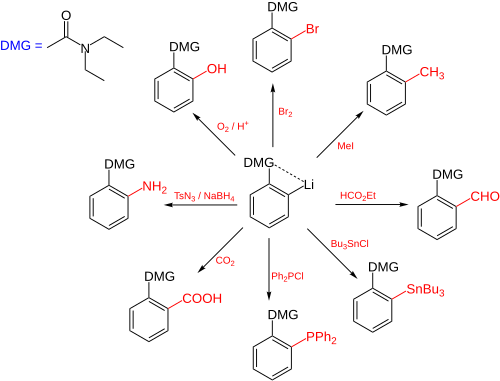
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound.[1] The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.The compound can be produced by directed lithiation of anisole.[2]

The general principle is outlined in scheme 1. An aromatic ring system with a DMG group 1 interacts with an alkyllithium such as n-butyllithium in its specific aggregation state (hence (R-Li)n) to intermediate 2 since the hetero atom on the DMG is a Lewis base and lithium the Lewis acid. The very basic alkyllithium then deprotonates the ring in the nearest ortho- position forming the aryllithium 3 all the while maintaining the acid-base interaction. An electrophile reacts in the next phase in an electrophilic aromatic substitution with a strong preference for the lithium ipso position replacing the lithium atom.
Ordinary electrophilic substitutions with an activating group show preference for both the ortho and para position, this reaction demonstrates increased regioselectivity because the ortho position alone is targeted.
This reaction type was reported independently by Henry Gilman and Georg Wittig around 1940.[3][4]
- ^ Snieckus, Victor (September 1990). "Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics". Chemical Reviews. 90 (6): 879–933. doi:10.1021/cr00104a001.
- ^ Gschwend, Heinz W.; Rodriguez, Herman R. (1979). "Heteroatom-Facilitated Lithiations". Organic Reactions. pp. 1–360. doi:10.1002/0471264180.or026.01. ISBN 0471264180.
- ^ Relative Reactivities of Organometallic Compounds. XX.* Metalation Henry Gilman, Robert L. Bebb J. Am. Chem. Soc.; 1939; 61(1); 109-112. doi:10.1021/ja01870a037
- ^ G. Wittig et al. Chem. Ber. 1940, 73, 1197