| |

| |
| Names | |
|---|---|
| IUPAC name
Disiloxane
| |
| Other names
Disilyl ether
Disilyl oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DS DSE |
| ChEBI | |
| ChemSpider | |
| 1206 | |
| MeSH | Disiloxane |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6OSi2 | |
| Molar mass | 78.217 g·mol−1 |
| Appearance | Colorless gas |
| Boiling point | −15.2 °C (4.6 °F; 257.9 K) |
| 0.24 D | |
| Structure | |
| Orthorhombic | |
| Pmm2 | |
| Bent | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Dimethyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
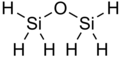
Disiloxane has the chemical formula Si
2H
6O. It is the simplest known siloxane with hydrogen only R groups. The molecule contains six equivalent Si−H bonds and two equivalent Si−O bonds. Disiloxane exists as a colorless, pungent gas under standard conditions. However, it is generally safe for human use as evidence in its widespread use in cosmetics. It is also commonly known as disilyl ether, disilyl oxide, and perhydrodisiloxane
