| |

| |
| Names | |
|---|---|
| IUPAC name
dithionic acid[1]
| |
| Other names
hypodisulfuric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
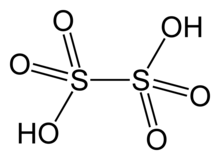
| H2S2O6 | |
| Molar mass | 162.14 g mol−1 |
| Acidity (pKa) | −3.4 (estimated)[2] |
| Conjugate base | Dithionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dithionic acid, H2S2O6, is the inorganic compound with the formula H2S2O6. It is the doubly protonated derivative of dithionate, a well-characterized dianion. Dithionic acid is mainly observed and characterized as an aqueous solution.[3]
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 63. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. pp. 715-716