 | |
| Clinical data | |
|---|---|
| Trade names | Dobutrex, Inotrex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682861 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, intraosseous[2] |
| Drug class | β1-agonist |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | Within 2 min[2] |
| Elimination half-life | 2 minutes |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
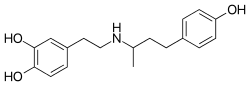
| Formula | C18H23NO3 |
| Molar mass | 301.386 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Dobutamine is a medication used in the treatment of cardiogenic shock (as a result of inadequate tissue perfusion) and severe heart failure.[2][3] It may also be used in certain types of cardiac stress tests.[2] It is given by IV only, as an injection into a vein or intraosseous as a continuous infusion.[2] The amount of medication needs to be adjusted to the desired effect.[2] Onset of effects is generally seen within 2 minutes.[2] It has a half-life of two minutes. This drug is generally only administered short term, although it may be used for longer periods to relieve symptoms of heart failure in patients awaiting heart transplantation.[4]
Common side effects include a fast heart rate, an irregular heart beat, and inflammation at the site of injection.[2][5] Use is not recommended in those with idiopathic hypertrophic subaortic stenosis.[2] It primarily works by direct stimulation of β1 receptors, which increases the strength of the heart's contractions, leading to a positive inotropic effect. Generally it has little effect on a person's heart rate.[2]
Dobutamine was approved for medical use in the United States in 1978.[2] It is available as a generic medication.[5] It was initially made from isoproterenol.[3]
- ^ a b "Dobutamine (Dobutrex) Use During Pregnancy". Drugs.com. 18 March 2020. Retrieved 17 May 2020.
- ^ a b c d e f g h i j k "Dobutamine Hydrochloride Monograph for Professionals". Drugs.com. AHFS. Retrieved 11 January 2019.
- ^ a b Wilson WC, Grande CM, Hoyt DB (2007). Trauma: Critical Care. CRC Press. p. 302. ISBN 978-1-4200-1684-0.
- ^ Gentile P, Marini C, Ammirati E, Perna E, Saponara G, Garascia A, et al. (October 2021). "Long-term administration of intravenous inotropes in advanced heart failure". ESC Heart Failure. 8 (5): 4322–4327. doi:10.1002/ehf2.13394. PMC 8497373. PMID 34191408.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 220–221. ISBN 978-0-85711-338-2.