| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(C20-Ih)[5]fullerane
hexadecahydro-1,6,5,2,4,3-(epibutane[1,1,2,3,4,4]hexayl)dipentaleno[2,1,6-gha:2′,1′,6′-cde]pentalene | |||
| Systematic IUPAC name
undecacyclo[9.9.0.02,9.03,7.04,20.05,18.06,16.08,15.010,14.012,19.013,17]icosane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1880116 | |||
| ChEBI | |||
| ChemSpider | |||
| 1326921 | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C20H20 | |||
| Molar mass | 260.380 g·mol−1 | ||
| Melting point | 430±10°C[1] | ||
| Related compounds | |||
Related hydrocarbons
|
Cubane Tetrahedrane Pagodane (an isomer of dodecahedrane) Prismane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
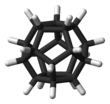
Dodecahedrane is a chemical compound, a hydrocarbon with formula C20H20, whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane.
Dodecahedrane does not occur in nature and has no significant uses. It was synthesized by Leo Paquette in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework".[2]
For many years, dodecahedrane was the simplest real carbon-based molecule with full icosahedral symmetry. Buckminsterfullerene (C60), discovered in 1985, also has the same symmetry, but has three times as many carbons and 50% more atoms overall. The synthesis of the C20 fullerene C20 in 2000, from brominated dodecahedrane,[3] may have demoted C20H20 to second place.
- ^ Lindberg, Thomas (2012-12-02). Strategies and Tactics in Organic Synthesis. ISBN 9780323152938.
- ^ Cite error: The named reference
paquette1982was invoked but never defined (see the help page). - ^ Cite error: The named reference
prinz2000was invoked but never defined (see the help page).