 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tivicay, Tivicay PD |
| Other names | GSK572, S-349572 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613043 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a[4] |
| Protein binding | ≥98.9% |
| Metabolism | UGT1A1 and CYP3A |
| Elimination half-life | ~14 hours |
| Excretion | Feces (53%) and urine (18.9%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.237.735 |
| Chemical and physical data | |
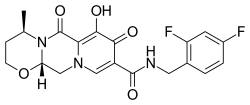
| Formula | C20H19F2N3O5 |
| Molar mass | 419.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS.[6] It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure.[7] It is taken by mouth.[6]
Common side effects include trouble sleeping, feeling tired, diarrhea, high blood sugar, and headache.[7] Severe side effects may include allergic reactions and liver problems.[7] Concerns that usage during pregnancy can result in harm to the baby have been refuted by further studies that show there is no statistical difference in neural tube defects from the usage of dolutegravir compared to other antiretrovirals.[8] It is unclear if use during breastfeeding is safe.[7] Dolutegravir is an HIV integrase strand transfer inhibitor which blocks the functioning of HIV integrase which is needed for viral replication.[7]
Dolutegravir was approved for medical use in the United States in 2013.[7] It is on the World Health Organization's List of Essential Medicines.[9] Abacavir/dolutegravir/lamivudine, a combination with abacavir and lamivudine is also available.[7][10][11] As of 2019, the World Health Organization (WHO) recommends DTG as the first- and second-line treatment for all persons with HIV.[12]
- ^ "Dolutegravir (Tivicay) Use During Pregnancy". Drugs.com. 16 October 2018. Retrieved 13 February 2020.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- ^ a b "Tivicay- dolutegravir sodium tablet, film coated". DailyMed. 24 October 2019. Retrieved 13 February 2020.
- ^ Cite error: The named reference
Tivicay EPARwas invoked but never defined (see the help page). - ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 429. ISBN 9780857111562.
- ^ a b c d e f g "Dolutegravir Sodium Monograph for Professionals". Drugs.com. Retrieved 20 April 2019.
- ^ Gill MM, Khumalo P, Chouraya C, Kunene M, Dlamini F, Hoffman HJ; et al. (2023). "Strengthening the Evidence: Similar Rates of Neural Tube Defects Among Deliveries Regardless of Maternal HIV Status and Dolutegravir Exposure in Hospital Birth Surveillance in Eswatini". Open Forum Infect Dis. 10 (9): ofad441. doi:10.1093/ofid/ofad441. PMC 10502921. PMID 37720700.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Ciccullo A, Baldin G, Borghetti A, Di Giambenedetto S (April 2020). "Dolutegravir plus lamivudine for the treatment of HIV-1 infection". Expert Review of Anti-infective Therapy. 18 (4): 279–292. doi:10.1080/14787210.2020.1729742. PMID 32067525. S2CID 211160876.
- ^ Patel R, Evitt L, Mariolis I, Di Giambenedetto S, d'Arminio Monforte A, Casado J, et al. (August 2021). "HIV Treatment with the Two-Drug Regimen Dolutegravir Plus Lamivudine in Real-world Clinical Practice: A Systematic Literature Review". Infectious Diseases and Therapy. 10 (4): 2051–2070. doi:10.1007/s40121-021-00522-7. PMC 8572911. PMID 34426899.
- ^ "WHO recommends dolutegravir as preferred HIV treatment option in all populations". World Health Organization (Press release). Retrieved 22 July 2019.