 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Motilium, others |
| Other names | R-33812; R33812; KW-5338; KW5338; NSC-299589; NSC299589 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal[2] |
| Drug class | D2 receptor antagonist; Prolactin releaser |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 13–17%[2][6] Intramuscular: 90%[2] |
| Protein binding | ~92%[2] |
| Metabolism | Hepatic (CYP3A4/5) and intestinal (first-pass)[2][7] |
| Metabolites | All inactive[2][7] |
| Onset of action | 30–60 minutes[8] |
| Elimination half-life | 7–9 hours[9][2][6] |
| Excretion | Feces: 66%[2] Urine: 32%[2] Breast milk: small quantities[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.408 |
| Chemical and physical data | |
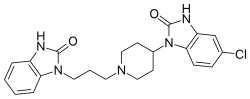
| Formula | C22H24ClN5O2 |
| Molar mass | 425.92 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 242.5 °C (468.5 °F) |
| |
| |
| (verify) | |
Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis (delayed gastric emptying). It raises the level of prolactin in the human body and is used off label to induce and promote breast milk production.[2][10] It may be taken by mouth or rectally.[2][11][12]
Side effects may include headache, anxiety, dry mouth, abdominal cramps, diarrhea, and elevated prolactin levels.[13][2][10][14] Secondary to increased prolactin levels, breast changes, milk outflow, menstrual irregularities, and hypogonadism can occur.[2][10][14] Domperidone may also cause QT prolongation and has rarely been associated with serious cardiac complications such as sudden cardiac death.[15][16][17][18] However, the risks are small and occur more with high doses.[18][19] Domperidone acts as a peripherally selective antagonist of the dopamine D2 and D3 receptors.[2][10] Due to its low entry into the brain, the side effects of domperidone are different from those of other dopamine receptor antagonists like metoclopramide and it produces little in the way of central nervous system adverse effects.[2][10] However, domperidone can nonetheless increase prolactin levels as the pituitary gland is outside of the blood–brain barrier.[20]
Domperidone was discovered in 1974 and was introduced for medical use in 1979.[21][22][23] It was developed by Janssen Pharmaceutica.[21][22] Domperidone is available over-the-counter in many countries, for instance in Europe and elsewhere throughout the world.[24][2] It is not approved for use in the United States.[25][26][2] However, it is available in the United States for people with severe and treatment-refractory gastrointestinal motility problems under an expanded access individual-patient investigational new drug application.[25] An analogue of domperidone called deudomperidone is under development for potential use in the United States and other countries.[27][28][29]
- ^ "Motilium Product Information". Therapeutic Goods Administration (TGA). 10 June 2024. Retrieved 10 June 2024.
- ^ a b c d e f g h i j k l m n o p q r Reddymasu SC, Soykan I, McCallum RW (September 2007). "Domperidone: review of pharmacology and clinical applications in gastroenterology". The American Journal of Gastroenterology. 102 (9): 2036–2045. doi:10.1111/j.1572-0241.2007.01255.x. PMID 17488253. S2CID 22575456.
- ^ "Motilium product information". Health Canada. 7 January 2002. Retrieved 10 June 2024.
- ^ "Motilium Summary of Product Characteristics (SmPC)". (emc). 11 March 2024. Retrieved 10 June 2024.
- ^ "Motilium referral". European Medicines Agency (EMA). 31 October 2000. Retrieved 10 June 2024.
- ^ a b Rose S (October 2004). Gastrointestinal and Hepatobiliary Pathophysiology. Hayes Barton Press. pp. 523–. ISBN 978-1-59377-181-2.[permanent dead link]
- ^ a b Simard C, Michaud V, Gibbs B, Massé R, Lessard E, Turgeon J (2008). "Identification of the cytochrome P450 enzymes involved in the metabolism of domperidone". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 34 (11–12): 1013–1023. doi:10.1080/00498250400015301. PMID 15801545. S2CID 27426219.
- ^ "Domperidone: Anti-sickness medicine used to treat nausea and vomiting". 7 January 2020.
- ^ Cite error: The named reference
EMC-Domperidonewas invoked but never defined (see the help page). - ^ a b c d e Barone JA (April 1999). "Domperidone: a peripherally acting dopamine2-receptor antagonist". The Annals of Pharmacotherapy. 33 (4): 429–440. doi:10.1345/aph.18003. PMID 10332535. S2CID 39279569.
- ^ Cite error: The named reference
emawas invoked but never defined (see the help page). - ^ "Motilium INSTANTS PL 13249/0028" (PDF). Medicines and Healthcare products Regulatory Agency. 23 February 2010. Archived from the original (PDF) on 13 December 2014. Retrieved 31 October 2014.
- ^ BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 444. ISBN 9780857113658.
- ^ a b Henderson A (2003). "Domperidone. Discovering new choices for lactating mothers". AWHONN Lifelines. 7 (1): 54–60. doi:10.1177/1091592303251726. PMID 12674062.
- ^ Cite error: The named reference
pmid26649742was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid20394569was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid24147629was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
pmid26117678was invoked but never defined (see the help page). - ^ Cite error: The named reference
MarziWeitz2015was invoked but never defined (see the help page). - ^ Paul C, Zénut M, Dorut A, Coudoré MA, Vein J, Cardot JM, et al. (February 2015). "Use of domperidone as a galactagogue drug: a systematic review of the benefit-risk ratio". Journal of Human Lactation. 31 (1): 57–63. doi:10.1177/0890334414561265. PMID 25475074. S2CID 7978585.
- ^ a b Cite error: The named reference
pmid18507654was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
ddwas invoked but never defined (see the help page). - ^ Cite error: The named reference
WilliamAndrewPublishing2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
FaisVermiglio2015was invoked but never defined (see the help page). - ^ a b "How to Request Domperidone for Expanded Access Use". U.S. Food and Drug Administration (FDA). 12 December 2023.
- ^ Cite error: The named reference
pmid15451832was invoked but never defined (see the help page). - ^ "Deudomperidone - CinRx Pharma - AdisInsight".
- ^ Heckroth M, Luckett RT, Moser C, Parajuli D, Abell TL (April 2021). "Nausea and Vomiting in 2021: A Comprehensive Update". Journal of Clinical Gastroenterology. 55 (4): 279–299. doi:10.1097/MCG.0000000000001485. PMC 7933092. PMID 33471485.
- ^ Wo JM, McCallum RW, Gonzalez Z (2021). "Antiemetic therapy for gastroparesis". Gastroparesis. Elsevier. pp. 341–359. doi:10.1016/B978-0-12-818586-5.00025-9. ISBN 9780128185865. S2CID 225132800.