 | |
| Clinical data | |
|---|---|
| Trade names | Aricept, Adlarity, others |
| Other names | Donepezil hydrochloride (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697032 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal |
| Drug class | Acetylcholinesterase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100%[5][6] |
| Protein binding | 96%, albumin (about 75%) and alpha1-acid glycoprotein (21%).[6][5] |
| Metabolism | CYP2D6, CYP3A4, and glucuronidation.[5] Four major metabolites, two of which are active.[6][5] |
| Onset of action | Peak plasma levels in 3–4 h.[6][5] |
| Elimination half-life | 70 hours[7] Around 100 hours in elderly patients.[5] |
| Duration of action | With daily dosing, steady-state concentration is reached in 15–21 days.[6][5] |
| Excretion | 0.11–0.13 (L/h/kg); excreted mostly by the kidneys. Around 17% is excreted unchanged in the urine. About 15% to 20% is excreted in feces.[5][6] Steady-state clearance is similar at all ages.[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.198 |
| Chemical and physical data | |
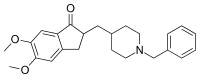
| Formula | C24H29NO3 |
| Molar mass | 379.500 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Donepezil, sold under the brand name Aricept among others, is a medication used to treat dementia of the Alzheimer's type.[3][4][8] It appears to result in a small benefit in mental function and ability to function.[9] Use, however, has not been shown to change the progression of the disease.[10] Treatment should be stopped if no benefit is seen.[11] It is taken by mouth or via a transdermal patch.[3][4][8]
Common side effects include nausea, trouble sleeping, aggression, diarrhea, feeling tired, and muscle cramps.[8][11] Serious side effects may include abnormal heart rhythms, urinary incontinence, and seizures.[8] Donepezil is a centrally acting reversible acetylcholinesterase inhibitor and structurally unrelated to other anticholinesterase agents.[8][5]
Donepezil was approved for medical use in the United States in 1996.[8] It is available as a generic medication.[11] In 2022, it was the 146th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[12][13]
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Donepezil Hydrochloride 10 mg Film-coated tablets". Electronic Medicines Compendium. Archived from the original on 8 September 2022. Retrieved 8 September 2022.
- ^ a b c "Aricept- donepezil hydrochloride tablet, film coated Aricept ODT- donepezil hydrochloride tablet, orally disintegrating". DailyMed. 23 December 2021. Retrieved 15 March 2022.
- ^ a b c "Adlarity- donepezil hydrochloride patch". DailyMed. 11 March 2022. Retrieved 19 June 2022.
- ^ a b c d e f g h i j Cite error: The named reference
Kum2020was invoked but never defined (see the help page). - ^ a b c d e f Seltzer B (October 2005). "Donepezil: a review". Expert Opinion on Drug Metabolism & Toxicology. 1 (3). Informa Healthcare: 527–536. doi:10.1517/17425255.1.3.527. PMID 16863459. S2CID 32689288.
there is a linear relationship between dose and pharmacodynamic effects, measured as red blood cell acetylcholinesterase inhibition and clinical efficacy. Despite being 96% bound to plasma proteins, it has few interactions with other drugs, and the 5-mg dose can be given safely to patients with mild-to-moderate hepatic and renal-disease.
- ^ Asiri YA, Mostafa GA (2010). "Donepezil". Profiles of Drug Substances, Excipients and Related Methodology. Vol. 35. Elsevier. pp. 117–50. doi:10.1016/s1871-5125(10)35003-5. ISBN 978-0-12-380884-4. ISSN 1871-5125. PMID 22469221. S2CID 206178636.
Plasma donepezil concentrations decline with a half-life of approximately 70 h. Sex, race, and smoking history have no clinically significant influence on plasma concentrations of donepezil [46–51].
{{cite book}}:|journal=ignored (help) - ^ a b c d e f "Donepezil Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 4 February 2019.
- ^ Birks JS, Harvey RJ (June 2018). "Donepezil for dementia due to Alzheimer's disease". The Cochrane Database of Systematic Reviews. 2018 (6): CD001190. doi:10.1002/14651858.CD001190.pub3. PMC 6513124. PMID 29923184.
- ^ Swedish Council on Health Technology Assessment (June 2008). "Dementia – Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review". SBU Systematic Reviews. PMID 28876770.
- ^ a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 300. ISBN 978-0-85711-338-2.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Donepezil Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.