 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksɪˈsaɪkliːn/ DOKS-iss-EYE-kleen |
| Trade names | Doxy, Doryx, Vibramycin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682063 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 80–90% |
| Metabolism | Negligible |
| Elimination half-life | 10–22 hours |
| Excretion | Mainly feces, 40% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.429 |
| Chemical and physical data | |
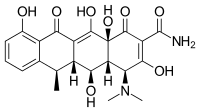
| Formula | C22H24N2O8 |
| Molar mass | 444.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Doxycycline is a broad-spectrum antibiotic of the tetracycline class used in the treatment of infections caused by bacteria and certain parasites.[1] It is used to treat bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, and syphilis.[1] It is also used to prevent malaria.[2][3] Doxycycline may be taken by mouth or by injection into a vein.[1]
Common side effects include diarrhea, nausea, vomiting, abdominal pain, and an increased risk of sunburn.[1] Use during pregnancy is not recommended.[1] Like other agents of the tetracycline class, it either slows or kills bacteria by inhibiting protein production.[1][4] It kills malaria by targeting a plastid organelle, the apicoplast.[5][6]
Doxycycline was patented in 1957 and came into commercial use in 1967.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] Doxycycline is available as a generic medicine.[1][10] In 2022, it was the 68th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[11][12]
- ^ a b c d e f g h "Doxycycline calcium". The American Society of Health-System Pharmacists. Archived from the original on 23 September 2015. Retrieved 18 August 2015.
- ^ "Malaria". Fit for Travel. Public Health Scotland. Chemoprophylaxis. Archived from the original on 4 December 2023. Retrieved 4 December 2023.
- ^ "Choosing a Drug to Prevent Malaria". Centers for Disease Control and Prevention. February 2023. Doxycycline. Archived from the original on 13 November 2016. Retrieved 4 December 2023.
- ^ Cite error: The named reference
pmid22191524was invoked but never defined (see the help page). - ^ McFadden GI (March 2014). "Apicoplast". Current Biology. 24 (7): R262-3. Bibcode:2014CBio...24.R262M. doi:10.1016/j.cub.2014.01.024. PMID 24698369.
- ^ Schlagenhauf-Lawlor P (2008). Travelers' Malaria. PMPH-USA. p. 148. ISBN 978-1-55009-336-0.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 978-3-527-60749-5.
- ^ Corey EJ (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 406. ISBN 978-1-118-35446-9. Archived from the original on 14 January 2023. Retrieved 9 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton RJ (2011). Tarascon pharmacopoeia (12th ed.). Sudbury, MA: Jones & Bartlett Learning. p. 79. ISBN 978-1-4496-0067-9.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Doxycycline Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Archived from the original on 8 July 2020. Retrieved 30 August 2024.