 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɪˈfævɪrɛnz/ i-FAV-i-renz |
| Trade names | Sustiva, Stocrin, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699004 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40–45% (under fasting conditions) |
| Protein binding | 99.5–99.75% |
| Metabolism | Liver (CYP2A6 and CYP2B6-mediated) |
| Onset of action | 3–5 hours |
| Elimination half-life | 40–55 hours |
| Excretion | Kidney (14–34%) and feces (16–61%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.346 |
| Chemical and physical data | |
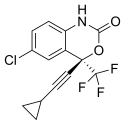
| Formula | C14H9ClF3NO2 |
| Molar mass | 315.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS.[1] It is generally recommended for use with other antiretrovirals.[1] It may be used for prevention after a needlestick injury or other potential exposure.[1] It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir.[1] It is taken by mouth.[1]
Common side effects include rash, nausea, headache, feeling tired, and trouble sleeping.[1] Some of the rashes may be serious such as Stevens–Johnson syndrome.[1] Other serious side effects include depression, thoughts of suicide, liver problems, and seizures.[1] It is not safe for use during pregnancy.[1] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and works by blocking the function of reverse transcriptase.[1]
Efavirenz was approved for medical use in the United States in 1998,[1] and in the European Union in 1999.[4] It is on the World Health Organization's List of Essential Medicines.[5] As of 2016, it is available as a generic medication.[6][7]
- ^ a b c d e f g h i j k l "Efavirenz". The American Society of Health-System Pharmacists. Archived from the original on 17 November 2016. Retrieved 28 November 2016.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 3 April 2024.
- ^ Cite error: The named reference
Sustiva FDA labelwas invoked but never defined (see the help page). - ^ a b "Stocrin EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 15 October 2020.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Cite error: The named reference
Efavirenz Drug Profilewas invoked but never defined (see the help page). - ^ Cite error: The named reference
Efavirenz generic approvalwas invoked but never defined (see the help page).