 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vaniqa, Iwilfin, others |
| Other names | α-difluoromethylornithine or DFMO |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (Intravenous) Negligible (topical) |
| Metabolism | Not metabolized |
| Elimination half-life | 8 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
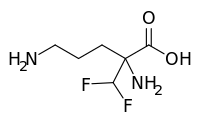
| Formula | C6H12F2N2O2 |
| Molar mass | 182.171 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Eflornithine, sold under the brand name Vaniqa among others, is a medication used to treat African trypanosomiasis (sleeping sickness) and excessive hair growth on the face in women.[1][3][4] Specifically it is used for the second stage of sleeping sickness caused by T. b. gambiense and may be used with nifurtimox.[3][5] It is taken intravenously (injection into a vein) or topically.[3][4] It is an ornithine decarboxylase inhibitor.[2]
Common side effects when applied as a cream include rash, redness, and burning.[4] Side effects of the injectable form include bone marrow suppression, vomiting, and seizures.[5] It is unclear if it is safe to use during pregnancy or breastfeeding.[5] It is recommended typically for children over the age of 12.[5]
Eflornithine was developed in the 1970s and came into medical use in 1990.[6] It is on the World Health Organization's List of Essential Medicines.[7] In the United States the injectable form can be obtained from the US Centers for Disease Control and Prevention.[5] In regions of the world where the disease is common eflornithine is provided for free by the World Health Organization.[8]
- ^ a b "Vaniqa- eflornithine hydrochloride cream". DailyMed. 18 September 2012. Retrieved 26 February 2024.
- ^ a b "Iwilfin- eflornithine hydrochloride tablet". DailyMed. 21 December 2023. Retrieved 26 February 2024.
- ^ a b c "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Archived (PDF) from the original on 13 May 2015. Retrieved 10 May 2015.
- ^ a b c "Eflornithine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- ^ a b c d e "CDC - African Trypanosomiasis - Resources for Health Professionals". U.S. Centers for Disease Control and Prevention (CDC). 10 August 2016. Archived from the original on 28 November 2016. Retrieved 6 December 2016.
- ^ Steverding D (2016). "Sleeping Sickness and Nagana Disease Caused by Trypanosoma brucei". In Marcondes CB (ed.). Arthropod Borne Diseases. Springer. p. 292. ISBN 9783319138848. Archived from the original on 10 September 2017.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Trypanosomiasis, human African (sleeping sickness)". World Health Organization. February 2016. Archived from the original on 4 December 2016. Retrieved 7 December 2016.