This article needs additional citations for verification. (January 2023) |
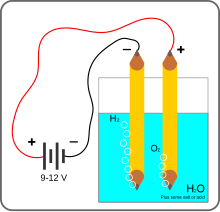

Electrolysis of water is using electricity to split water into oxygen (O
2) and hydrogen (H
2) gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Water electrolysis requires a minimum potential difference of 1.23 volts, although at that voltage external heat is also required. Typically 1.5 volts is required. Electrolysis is rare in industrial applications since hydrogen can be produced less expensively from fossil fuels.[1] Most of the time, hydrogen is made by splitting methane (CH4) into carbon dioxide (CO2) and hydrogen (H2). It’s a carbon-intensive process that means for every kilogram of “grey” hydrogen produced, approximately 10 kilograms of CO2 are emitted into the atmosphere.[2]
- ^ "Hydrogen Basics — Production". Florida Solar Energy Center. 2007. Archived from the original on 18 February 2008. Retrieved 5 February 2008.
- ^ Cuff, Madeleine (28 August 2024). "Is ultra cheap green hydrogen on the horizon?". New Scientist. Retrieved 29 August 2024.