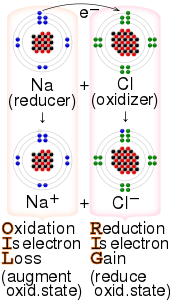
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.[2]
Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes.[3][4] In organic chemistry ET is a step in some industrial polymerization reactions. It is foundational to photoredox catalysis.
- ^ "Metals". Bitesize. BBC. Archived from the original on 2022-11-03.
- ^ Piechota, Eric J.; Meyer, Gerald J. (2019). "Introduction to Electron Transfer: Theoretical Foundations and Pedagogical Examples". Journal of Chemical Education. 96 (11): 2450–2466. Bibcode:2019JChEd..96.2450P. doi:10.1021/acs.jchemed.9b00489. S2CID 208754569.
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.