| |

| |
| Names | |
|---|---|
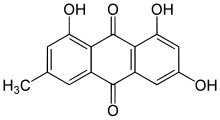
| Preferred IUPAC name
1,3,8-Trihydroxy-6-methylanthracene-9,10-dione | |
| Other names
6-Methyl-1,3,8-trihydroxyanthraquinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.509 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Appearance | Orange solid |
| Density | 1.583±0.06 g/cm3 |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is an organic compound. Classified as an anthraquinone,it can be isolated from rhubarb, buckthorn, and Japanese knotweed (Reynoutria japonica syn. Polygonum cuspidatum).[1] Emodin is particularly abundant in the roots of the Chinese rhubarb (Rheum palmatum), knotweed and knotgrass (Polygonum cuspidatum and Polygonum multiflorum) as well as Hawaii ‘au‘auko‘i cassia seeds or coffee weed (Semen cassia).[2] It is specifically isolated from Rheum palmatum L.[3] It is also produced by many species of fungi, including members of the genera Aspergillus, Pyrenochaeta, and Pestalotiopsis, inter alia. The common name is derived from Rheum emodi, a taxonomic synonym of Rheum australe, (Himalayan rhubarb) and synonyms include emodol, frangula emodin, rheum emodin, 3-methyl-1,6,8-trihydroxyanthraquinone, Schüttgelb (Schuttgelb), and Persian Berry Lake.[4]
- ^ Dorland's Medical Dictionary (1938)
- ^ Dellafiora L, Dorne JL, Galaverna G, Dall'Asta C (2020). "Preventing the Interaction between Coronaviruses Spike Protein and Angiotensin I Converting Enzyme 2: An in Silico Mechanistic Case Study on Emodin as a Potential Model Compound". Applied Sciences. 10 (18): 6358. doi:10.3390/app10186358. S2CID 224994102.
- ^ Tsay HS, Shyur LF, Agrawal DC, Wu YC, Wang SY (3 November 2017). Medicinal Plants – Recent Advances in Research and Development. Singapore: Springer Singapore. p. 339. ISBN 978-981-10-5978-0.
- ^ CID 3220 from PubChem