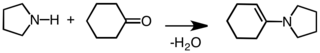An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine.[1][2] Enamines are versatile intermediates.[3][4]
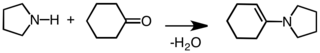
Condensation to give an enamine.[5]
The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group containing both alkene (en-) and alcohol (-ol). Enamines are considered to be nitrogen analogs of enols.[6]
If one or both of the nitrogen substituents is a hydrogen atom it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom.
Enamines are both good nucleophiles and good bases. Their behavior as carbon-based nucleophiles is explained with reference to the following resonance structures.
- ^ Clayden, Jonathan (2001). Organic chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 978-0-19-850346-0.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Enamines: Synthesis: Structure, and Reactions, Second Edition, Gilbert Cook (Editor). 1988, Marcel Dekker, NY. ISBN 0-8247-7764-6
- ^ R. B. Woodward, I. J. Pachter, and M. L. Scheinbaum (1974). "2,2- (Trimethylenedithio)cyclohexanone". Organic Syntheses. 54: 39
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 5, p. 1014. - ^ R. D. Burpitt and J. G. Thweatt (1968). "Cyclodecanone". Organic Syntheses. 48: 56; Collected Volumes, vol. 5, p. 277.
- ^ Imines and Enamines | PharmaXChange.info