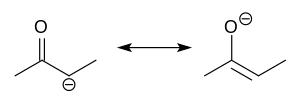
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl (RR'C=O) compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.[1][2][3][4]
- ^ Stolz, Daniel; Kazmaier, Uli (2010). "Metal Enolates as Synthons in Organic Chemistry". PATai's Chemistry of Functional Groups. doi:10.1002/9780470682531.pat0423. ISBN 978-0-470-68253-1.
- ^ Hart, David J.; Ha, Deok Chan (1989). "The ester enolate-imine condensation route to .beta.-lactams". Chemical Reviews. 89 (7): 1447–1465. doi:10.1021/cr00097a003.
- ^ Wu, George; Huang, Mingsheng (2006). "Organolithium Reagents in Pharmaceutical Asymmetric Processes". Chemical Reviews. 106 (7): 2596–2616. doi:10.1021/cr040694k. PMID 16836294.
- ^ Curti, Claudio; Battistini, Lucia; Sartori, Andrea; Zanardi, Franca (2020). "New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems". Chemical Reviews. 120 (5): 2448–2612. doi:10.1021/acs.chemrev.9b00481. PMC 7993750. PMID 32040305.