| |
| Names | |
|---|---|
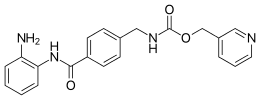
| Preferred IUPAC name
(Pyridin-3-yl)methyl ({4-[(2-aminophenyl)carbamoyl]phenyl}methyl)carbamate | |
| Other names
SNDX-275; MS-275
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.158.999 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20N4O3 | |
| Molar mass | 376.4085 g/mol |
| Pharmacology | |
| L01XH05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Entinostat, also known as SNDX-275 and MS-275, is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers.[1][2][3][4]
Entinostat inhibits class I HDAC1 and HDAC3 with IC50 of 0.51 μM and 1.7 μM, respectively.[5]
Syndax pharmaceuticals currently holds the rights to entinostat and recently received $26.6 million in funds to advance treatments of resistant cancers using epigenetic tools.[6]
It has also been investigated as a potential male contraceptive drug.[7]
- ^ Juergens RA, Vendetti F, Coleman B, Sebree RS, Rudek MA, Belinsky SA, et al. (May 2008). "Phase I trial of 5-azacitidine (5AC) and SNDX-275 in advanced lung cancer (NSCLC)". Journal of Clinical Oncology. 26 (15_suppl): 19036-. doi:10.1200/jco.2008.26.15_suppl.19036.
- ^ Kiany S, Harrison D, Gordon N (2020). "The Histone Deacetylase Inhibitor Entinostat/Syndax 275 in Osteosarcoma". Current Advances in Osteosarcoma. Advances in Experimental Medicine and Biology. Vol. 1257. pp. 75–83. doi:10.1007/978-3-030-43032-0_7. ISBN 978-3-030-43031-3. PMID 32483732. S2CID 219169967.
- ^ Wang Y, Xie Q, Tan H, Liao M, Zhu S, Zheng LL, et al. (November 2021). "Targeting cancer epigenetic pathways with small-molecule compounds: Therapeutic efficacy and combination therapies". Pharmacological Research. 173: 105702. doi:10.1016/j.phrs.2021.105702. PMID 34102228. S2CID 235378858.
- ^ Lian B, Chen X, Shen K (2023). "Inhibition of histone deacetylases attenuates tumor progression and improves immunotherapy in breast cancer". Frontiers in Immunology. 14: 1164514. doi:10.3389/fimmu.2023.1164514. PMC 10034161. PMID 36969235.
- ^ US 2009/0263353, Maier T, Beckers T, Hummel RP, Feth M, Muller M, Bar T, Volz J, "Novel Sulphonylpyrroles as Inhibitors of Hdac S Novel Sulphonylpyrroles", issued 31 July 2012, assigned to 4SC AG
- ^ "Company Prepares for Pivotal Phase 3 Study of Entinostat, Most Advanced HDAC Inhibitor in Development for ER+ Metastatic Breast Cancer" (PDF). Syndax Pharmaceuticals. 27 August 2013. Archived from the original (PDF) on 17 June 2016.
- ^ Hong SH, Castro G, Wang D, Nofsinger R, Kane M, Folias A, et al. (February 2024). "Targeting nuclear receptor corepressors for reversible male contraception". Proceedings of the National Academy of Sciences of the United States of America. 121 (9): e2320129121. doi:10.1073/pnas.2320129121. PMID 38377195.