| Epothilones | |
|---|---|

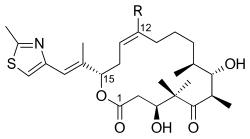
Epothilones A (R = H) and B (R = CH3) | |
| Chemical formulae |
A: C26H39NO6S |
| Molecular masses |
A: 493.66 g/mol |
| CAS numbers |
A: 152044-53-6 |
| PubChem |
A: 448799 |
Epothilones C (R = H) and D (R = CH3) | |
| Chemical formulae |
C: C26H39NO5S |
| Molecular masses |
C: 477.66 g/mol |
| CAS numbers |
C: 186692-73-9 |
| PubChem |
C: 9891226 |

Epothilones E (R = H) and F (R = CH3) | |
| Chemical formulae |
E: C26H39NO7S |
| Molecular masses |
E: 509.66 g/mol |
| CAS numbers |
E: 201049-37-8 |
| PubChem |
E: 9806341 |
| Disclaimer and references | |
Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.[1][2]
Epothilones were originally identified as metabolites produced by the soil-dwelling myxobacterium Sorangium cellulosum.[3] As of September 2008[update], epothilones A to F have been identified and characterized.[4]
Early studies in cancer cell lines and human cancer patients indicate superior efficacy to the taxanes. Their mechanism of action is similar, but their chemical structure is simpler. Due to their better water solubility, cremophors (solubilizing agents used for paclitaxel which can affect cardiac function and cause severe hypersensitivity) are not needed.[5] Endotoxin-like properties known from paclitaxel, like activation of macrophages synthesizing inflammatory cytokines and nitric oxide, are not observed for epothilone B.[6]
- ^ Rosenberg, Steven; DeVita, Vincent T.; Hellman, Samuel (2005). Cancer: Principles & Practice of Oncology (7th ed.). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-4450-4.
- ^ Forli, Stefano (2014). "Epothilones: from discovery to clinical trials". Current Topics in Medicinal Chemistry. 14 (20): 2312–2321. doi:10.2174/1568026614666141130095855. PMC 4629788. PMID 25434353.
- ^ "Epothilone - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2022-06-18.
- ^ H. Spreitzer (September 15, 2008). "Neue Wirkstoffe – Sagobepilon – eine synthetische Variation von Epothilon B als Hoffnungsträger gegen Krebs". Österreichische Apothekerzeitung (in German) (19/2008): 978.
- ^ Julien, B.; Shah, S. (2002). "Heterologous Expression of Epothilone Biosynthetic Genes in Myxococcus xanthus". Antimicrob. Agents Chemother. 46 (9): 2772–8. doi:10.1128/AAC.46.9.2772-2778.2002. PMC 127399. PMID 12183227.
- ^ Muhlradt, P.F.; Sasse, F. (1997). "Epothilone B stabilizes microtubuli of macrophages like taxol without showing taxol-like endotoxin activity". Cancer Research. 57 (16): 3344–6. PMID 9269992.