This article needs additional citations for verification. (November 2016) |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Integrilin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601210 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | ~25% |
| Elimination half-life | ~2.5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.160 |
| Chemical and physical data | |
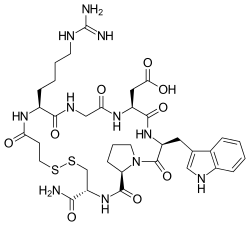
| Formula | C35H49N11O9S2 |
| Molar mass | 831.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Eptifibatide (Integrilin, Millennium Pharmaceuticals, also co-promoted by Schering-Plough/Essex), is an antiplatelet drug of the glycoprotein IIb/IIIa inhibitor class.[1] Eptifibatide is a cyclic heptapeptide derived from a disintegrin protein (P22827) found in the venom of the southeastern pygmy rattlesnake (Sistrurus miliarius barbouri). It belongs to the class of the arginin-glycin-aspartat-mimetics and reversibly binds to platelets. Eptifibatide has a short half-life. The drug is the third inhibitor of GPIIb/IIIa that has found broad acceptance after the specific antibody abciximab and the non-peptide tirofiban entered the global market.
- ^ Gribble GW (15 December 2010). Heterocyclic Scaffolds II: Indoles: Synthesis, Properties and Applications. Springer. pp. 11–. ISBN 978-3-642-15732-5. Retrieved 12 November 2010.