This article needs to be updated. (November 2018) |
| |
| Names | |
|---|---|
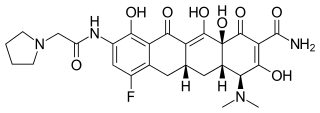
| Preferred IUPAC name
(4S,4aS,5aR,12aS)-4-(Dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-[2-(pyrrolidin-1-yl)acetamido]-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide | |
| Other names
Xerava
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H31FN4O8 | |
| Molar mass | 558.555 |
| Pharmacology | |
| J01AA13 (WHO) | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eravacycline (TP-434, Xerava) is a synthetic halogenated tetracycline class antibiotic by Tetraphase Pharmaceuticals. It is closely related to tigecycline. It has a broad spectrum of activity including many multi-drug resistant strains of bacteria. Phase III studies in complicated intra-abdominal infections (cIAI)[2] and complicated urinary tract infections (cUTI)[3] were recently completed with mixed results. Eravacycline was granted fast track designation by the FDA[4] and is currently available in USA.
- ^ "Xerava EPAR". European Medicines Agency. 8 October 2018. Retrieved 29 June 2024.
- ^ Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, et al. (16 November 2016). "Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial". JAMA Surgery. 152 (3): 224–232. doi:10.1001/jamasurg.2016.4237. ISSN 2168-6262. PMID 27851857. S2CID 42977246.
- ^ "Tetraphase Announces Top-Line Results From IGNITE2 Phase 3 Clinical Trial of Eravacycline in cUTI (NASDAQ:TTPH)". ir.tphase.com. Archived from the original on 21 November 2016. Retrieved 20 November 2016.
- ^ "FDA Grants QIDP Designation to Eravacycline, Tetraphase's Lead Antibiotic Product Candidate | Business Wire". businesswire.com. 15 July 2013. Retrieved 20 November 2016.