 | |
| Clinical data | |
|---|---|
| Trade names | Aptiom, Zebinix, Exalief |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~30%[5] |
| Metabolism | UGT (?) |
| Metabolites | Eslicarbazepine (active), glucuronides (inactive), etc. |
| Elimination half-life | 10–20 hours |
| Excretion | ~90% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.398 |
| Chemical and physical data | |
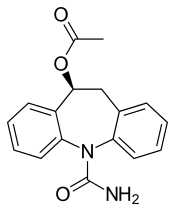
| Formula | C17H16N2O3 |
| Molar mass | 296.326 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is an anticonvulsant medication approved for use in Europe and the United States as monotherapy or as additional therapy for partial-onset seizures epilepsy.[6][4][3]
Similarly to oxcarbazepine, ESL behaves as a prodrug to (S)-(+)-licarbazepine.[7] As such, their mechanisms of action are identical.[8]
- ^ a b "Zebinix". Therapeutic Goods Administration (TGA). 9 June 2021. Retrieved 6 September 2021.
- ^ "Summary Basis of Decision (SBD) for Aptiom". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ a b "Aptiom- eslicarbazepine acetate tablet Aptiom- eslicarbazepine acetate kit". DailyMed. Retrieved 21 January 2021.
- ^ a b "Zebinix EPAR". European Medicines Agency (EMA). Retrieved 21 January 2021.
- ^ Dinnendahl V, Fricke U, eds. (2011). Arzneistoff-Profile (in German). Vol. 4 (25 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ "FDA approves Aptiom to treat seizures in adults". US FDA. 8 November 2013. Archived from the original on 11 January 2017. Retrieved 16 December 2019.
- ^ Rogawski MA (June 2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Research. 69 (3): 273–94. doi:10.1016/j.eplepsyres.2006.02.004. PMC 1562526. PMID 16621450.
- ^ Rogawski MA, Löscher W (July 2004). "The neurobiology of antiepileptic drugs". Nature Reviews. Neuroscience. 5 (7): 553–64. doi:10.1038/nrn1430. PMID 15208697. S2CID 2201038.