 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛsoʊˈmɛprəˌzoʊl, -ˈmiː-, -ˌzɒl/[1] |
| Trade names | Nexium, others[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699054 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Proton pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50 to 90% |
| Metabolism | Liver (CYP2C19, CYP3A4) |
| Elimination half-life | 1–1.5 hours |
| Excretion | 80% Kidney 20% Feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.048 |
| Chemical and physical data | |
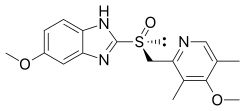
| Formula | C17H19N3O3S |
| Molar mass | 345.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Esomeprazole, sold under the brand name Nexium [or Neksium] among others,[2] is a medication which reduces stomach acid.[11] It is used to treat gastroesophageal reflux disease, peptic ulcer disease, and Zollinger–Ellison syndrome.[11][12] Its effectiveness is similar to that of other proton pump inhibitors (PPIs).[13] It is taken by mouth or injection into a vein.[11]
Common side effects include headache, constipation, dry mouth, and abdominal pain.[11] Serious side effects may include angioedema, Clostridioides difficile infection, and pneumonia.[11] Use in pregnancy appears to be safe, while safety during breastfeeding is unclear.[3] Esomeprazole is the (S)-(−)-enantiomer (or less specifically the S-isomer) of omeprazole.[11] It works by blocking H+/K+-ATPase in the parietal cells of the stomach.[11]
It was patented in 1993 and approved for medical use in 2000.[14] It is available as a generic medication and sold over the counter in several countries.[15][12] In 2022, it was the 122nd most commonly prescribed medication in the United States, with more than 5 million prescriptions.[16][17] In Australia, it was one of the top 10 most prescribed medications between 2017 and 2023.[18] It is also available in lower dose formulations without a prescription in the United States,[19] the United Kingdom[20] as well as Australia, Canada, and New Zealand.[21]
- ^ "Esomeprazole". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 21 January 2016.
- ^ a b "Esomeprazole Brand Names". BDdrugs.com. Bangladesh. 2011. Archived from the original on 7 February 2012.
- ^ a b "Esomeprazole Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ "Esomeprazole". Therapeutic Goods Administration (TGA). 15 September 2017. Retrieved 19 July 2020.
- ^ "Toreso/Esocolam/Meprator/Esotor/Nesed/Esome/Esotrack (Torrent Australasia Pty Ltd)". Therapeutic Goods Administration (TGA). 16 February 2023. Retrieved 9 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Nexium- esomeprazole magnesium capsule, delayed release Nexium- esomeprazole magnesium granule, delayed release". DailyMed. U.S. Library of Medicine. 7 June 2018. Retrieved 7 December 2020.
- ^ "Nexium 24HR- esomeprazole magnesium capsule, delayed release Nexium 24HR ClearMinis- esomeprazole magnesium capsule, delayed release". DailyMed. 26 May 2020. Retrieved 7 December 2020.
- ^ "Nexium I.V.- esomeprazole sodium injection". DailyMed. U.S. Library of Medicine. 27 November 2020. Retrieved 7 December 2020.
- ^ "Nexium Control EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 14 January 2021.
- ^ a b c d e f g "Esomeprazole Magnesium Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 78. ISBN 978-0-85711-338-2.
- ^ "[99] Comparative effectiveness of proton pump inhibitors | Therapeutics Initiative". 28 June 2016. Retrieved 14 July 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 445. ISBN 978-3-527-60749-5.
- ^ Jones & Bartlett Learning (2017). 2018 Nurse's Drug Handbook. Jones & Bartlett Learning. p. 394. ISBN 978-1-284-12134-6.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Esomeprazole Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Medicines in the health system". Australian Institute of Health and Welfare. 2 July 2024. Retrieved 30 September 2024.
- ^ Cite error: The named reference
USA2020OTCwas invoked but never defined (see the help page). - ^ "About esomeprazole". 13 January 2022.
- ^ "Australian TGA Scheduling of Esomeprazole". 15 September 2017. Retrieved 1 August 2023.