| |
| Names | |
|---|---|
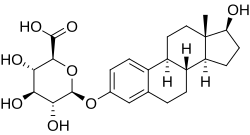
| IUPAC name
17β-Hydroxyestra-1,3,5(10)-trien-3-yl β-D-glucopyranosiduronic acid
| |
| Systematic IUPAC name
(2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-{[(1S,3aS,3bR,9bS,11aS)-1-hydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-7-yl]oxy}oxane-2-carboxylic acid | |
| Other names
E2-3G; 17β-Estradiol 3-(β-D-glucuronide)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H32O8 | |
| Molar mass | 448.512 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Estradiol 3-glucuronide (E2-3G), also known as 17β-estradiol 3-(β-D-glucuronide), is a naturally occurring and endogenous estrogen conjugate.[1] It is specifically the C3 glucuronide conjugate of estradiol, the major estrogen in the body.[1] It is formed from estradiol in the liver by UDP-glucuronosyltransferase via attachment of glucuronic acid and is eventually excreted in urine and bile.[2][3] Similarly to estrogen sulfates like estrone sulfate, estrogen glucuronides have much higher water solubility than do unconjugated estrogens like estradiol.[3]
Estrogen glucuronides can be deconjugated into the corresponding free estrogens by β-glucuronidase in tissues that express this enzyme, such as the mammary gland.[2] As a result, estrogen glucuronides have estrogenic activity via conversion into estrogens.[2]
Estradiol 3-glucuronide is a positional isomer of estradiol 17β-glucuronide.
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
- ^ a b "Human Metabolome Database: Showing metabocard for 17-beta-Estradiol-3-glucuronide (HMDB0006224)".
- ^ a b c Zhu BT, Conney AH (January 1998). "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis. 19 (1): 1–27. doi:10.1093/carcin/19.1.1. PMID 9472688.
- ^ a b Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.