 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lunesta, Eszop, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605009 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 52–59% |
| Metabolism | Liver oxidation and demethylation (CYP3A4 and CYP2E1-mediated) |
| Elimination half-life | 6 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.304 |
| Chemical and physical data | |
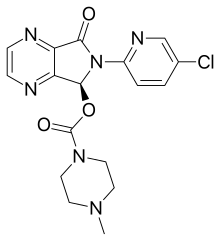
| Formula | C17H17ClN6O3 |
| Molar mass | 388.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Eszopiclone, sold under the brand name Lunesta among others, is a medication used in the treatment of insomnia.[3][4] Evidence supports slight to moderate benefit up to six months.[5][4][6] It is taken by mouth.[3][5]
Common side effects include headache, dry mouth, nausea, and dizziness.[5] Severe side effects may include suicidal thoughts, hallucinations, and angioedema.[5] Rapid decreasing of the dose may result in withdrawal.[5] Eszopiclone is classified as a nonbenzodiazepine or Z-drug and a sedative and hypnotic of the cyclopyrrolone group.[7] It is the S-stereoisomer of zopiclone.[5][8] It works by interacting with the GABA receptors.[7]
Approved for medical use in the United States in 2004,[3] eszopiclone is available as a generic medication.[5] In 2020, it was the 232nd most commonly prescribed medication in the United States, with more than 1 million prescriptions.[9][10] Eszopiclone is not sold in the European Union; as of 2009, the European Medicines Agency (EMA) ruled that it was too similar to zopiclone to be considered a new active substance.[11][12][13]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c d "Lunesta- eszopiclone tablet, coated". DailyMed. 24 May 2023. Retrieved 7 July 2023.
- ^ a b Cite error: The named reference
TB2012was invoked but never defined (see the help page). - ^ a b c d e f g "Eszopiclone Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 6 April 2019.
- ^ Rösner S, Englbrecht C, Wehrle R, Hajak G, Soyka M (October 2018). "Eszopiclone for insomnia". The Cochrane Database of Systematic Reviews. 2018 (10): CD010703. doi:10.1002/14651858.CD010703.pub2. PMC 6492503. PMID 30303519.
- ^ a b Cite error: The named reference
Davis2017was invoked but never defined (see the help page). - ^ Rösner S, Englbrecht C, Wehrle R, Hajak G, Soyka M (October 2018). "Eszopiclone for insomnia". The Cochrane Database of Systematic Reviews. 2018 (10): CD010703. doi:10.1002/14651858.CD010703.pub2. PMC 6492503. PMID 30303519.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Eszopiclone - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ "Lunivia: Withdrawn application". European Medicines Agency. 17 September 2018. Retrieved 19 February 2023.
- ^ Edwards J (13 June 2009). "End of Sepracor-GSK Deal Raises Question in Lunesta Patent Fight". www.cbsnews.com. Retrieved 7 April 2019.
- ^ "Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)". European Medicines Agency. 15 June 2009. Archived from the original on 3 August 2012. Retrieved 7 April 2019.