| |||
| |||
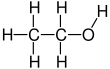
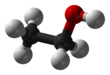
 Absolute ethanol
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈɛθənɒl/ | ||
| Preferred IUPAC name
Ethanol[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1718733 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.526 | ||
| 787 | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | UN 1170 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.069 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | wine-like, pungent[2] | ||
| Density | 0.78945 g/cm3 (at 20 °C)[3] | ||
| Melting point | −114.14 ± 0.03[3] °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.23 ± 0.09[3] °C (172.81 ± 0.16 °F; 351.38 ± 0.09 K) | ||
| Miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO)[4][5] | ||
| −33.60·10−6 cm3/mol | |||
Refractive index (nD)
|
1.3611[3] | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C)[6] | ||
| 1.69 D[7] | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H319, H360D | |||
| P210, P233, P240, P241, P242, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 14 °C (Absolute)[9] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1000 ppm (1900 mg/m3)[10] | ||
REL (Recommended)
|
TWA 1000 ppm (1900 mg/m3)[10] | ||
IDLH (Immediate danger)
|
3300 ppm [11] | ||
| Safety data sheet (SDS) | [8] | ||
| Related compounds | |||
Related compounds
|
|||
| Supplementary data page | |||
| Ethanol (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste.[13][14] In nature, grape-sugar breaks up by the action of fermentation into alcohol or carbonic acid, without anything being added.[15] As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine.[16]
Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning.[17][18] It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source for lamps, stoves, and internal combustion engines. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2023, world production of ethanol fuel was 29,590,000,000 US gallons (112.0 gigalitres), coming mostly from the U.S. (51%) and Brazil (26%).[19]
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge, UK: The Royal Society of Chemistry. 2014. p. 30. doi:10.1039/9781849733069-00001. ISBN 978-0-85404-182-4.
- ^ "Ethanol". PubChem. Retrieved 29 December 2022.
- ^ a b c d Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.246. ISBN 1-4398-5511-0.
- ^ Ballinger P, Long FA (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds1,2". Journal of the American Chemical Society. 82 (4): 795–798. doi:10.1021/ja01489a008. ISSN 0002-7863.
- ^ Arnett EM, Venkatasubramaniam KG (1983). "Thermochemical acidities in three superbase systems". J. Org. Chem. 48 (10): 1569–1578. doi:10.1021/jo00158a001.
- ^ Lide DR, ed. (2012). CRC Handbook of Chemistry and Physics (92 ed.). Boca Raton, FL: CRC Press/Taylor and Francis. pp. 6–232.
- ^ Lide DR, ed. (2008). CRC Handbook of Chemistry and Physics (89 ed.). Boca Raton, FL: CRC Press. pp. 9–55.
- ^ "MSDS Ethanol". Retrieved 12 January 2023.
- ^ "Ethanol". webwiser.nlm.nih.gov. Retrieved 25 June 2021.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0262". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Ethyl Alcohol". Retrieved 23 December 2023.
- ^ "Ethyl Alcohol". 2 November 2018. Retrieved 23 December 2023.
- ^ "Ethanol". PubChem. National Library of Medicine. Retrieved 28 September 2021.
- ^ "Ethyl Alcohol" (PDF). Hazardous Substance Fact Sheet. New Jersey Department of Health. Retrieved 28 September 2021.
- ^ Black (1875). Encyclopædia Britannica, Vol. 1. Edinburgh: Adam and Charles Black. p. 470.
- ^ Song, Frank; Walker, Matthew P. (8 November 2023). "Sleep, alcohol, and caffeine in financial traders". PLOS ONE. 18 (11): e0291675. Bibcode:2023PLoSO..1891675S. doi:10.1371/journal.pone.0291675. ISSN 1932-6203. PMC 10631622. PMID 37939019.
- ^ Powell MA (2004). "9: Wine and the vine in ancient Mesopotamia: the cuneiform evidence". In McGovern PE, Fleming SJ, Katz SH (eds.). The Origins and Ancient History of Wine. Food and Nutrition in History and Anthropology. Vol. 11 (1 ed.). Amsterdam: Taylor & Francis. pp. 96–124. ISBN 978-0-203-39283-6. ISSN 0275-5769. Retrieved 15 September 2010.
- ^ Schnelle, Norbert (August 1965). "Alcohol Given Intravenously for General Anesthesia". Surgical Clinics of North America. 45 (4): 1041–1049. doi:10.1016/S0039-6109(16)37650-2. PMID 14312998. Retrieved 30 December 2022.
- ^ "2008 World Fuel Ethanol Production". Ellisville, Missouri: Renewable Fuels Association. Retrieved 21 June 2024.