 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Intelence |
| Other names | TMC125 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Liver (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Elimination half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.546 |
| Chemical and physical data | |
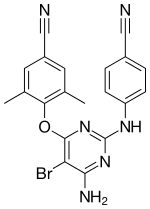
| Formula | C20H15BrN6O |
| Molar mass | 435.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etravirine (ETR,[3]), sold under the brand name Intelence is an antiretroviral medication used for the treatment of HIV.[1] Etravirine is a human immunodeficiency virus type 1 (HIV-1) non-nucleoside reverse transcriptase inhibitor (NNRTI).[1] Unlike agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine.[4] Etravirine is marketed by Janssen, a subsidiary of Johnson & Johnson. In January 2008, the US Food and Drug Administration (FDA) approved its use for people with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008.[5] It was also approved for use in Canada in April 2008.[6]
Etravirine is licensed in the United States, Canada, Israel, Russia, Australia, New Zealand, and the European Union,[7] and is under regulatory review in Switzerland.[8]
- ^ a b c "Intelence- etravirine tablet". DailyMed. 15 August 2023. Retrieved 14 August 2024.
- ^ "Intelence EPAR". European Medicines Agency (EMA). 28 August 2008. Retrieved 14 August 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Appendix A: Key to Acronyms". Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. Archived from the original on 31 August 2012.
- ^ Stellbrink HJ (October 2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". European Journal of Medical Research. 12 (9): 483–495. PMID 17933730.
- ^ "FDA Approves HIV Drug Etravirine". Associated Press. 18 January 2008.[dead link]
- ^ "First New NNRTI in Nearly a Decade to Benefit Canadians with HIV/AIDS" (PDF) (Press release). Janssen-Ortho Inc. 1 April 2008. Archived from the original (PDF) on 2 November 2010. Retrieved 9 July 2008.
- ^ "Intelence receives marketing authorisation in the European Union for HIV combination therapy". Tibotec. Archived from the original on 28 September 2011. Retrieved 29 August 2008.
- ^ "Etravirine (TMC125, Intelence) granted accelerated approval in US". aidsmap. Archived from the original on 2 January 2010. Retrieved 24 January 2008.