| FKBP-type peptidyl-prolyl cis-trans isomerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
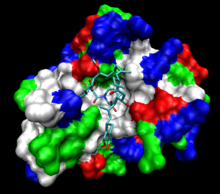 The human protein FKBP12 bound to FK506 (tacrolimus). The protein surface is colored by hydrophobicity; the deep cleft in which the ligand is bound is hydrophobic. | |||||||||
| Identifiers | |||||||||
| Symbol | FKBP_C | ||||||||
| Pfam | PF00254 | ||||||||
| InterPro | IPR001179 | ||||||||
| PROSITE | PDOC00426 | ||||||||
| SCOP2 | 1fkb / SCOPe / SUPFAM | ||||||||
| Membranome | 336 | ||||||||
| |||||||||

The FKBPs, or FK506 binding proteins, constitute a family of proteins that have prolyl isomerase activity and are related to the cyclophilins in function, though not in amino acid sequence.[1] FKBPs have been identified in many eukaryotes, ranging from yeast to humans, and function as protein folding chaperones for proteins containing proline residues. Along with cyclophilin, FKBPs belong to the immunophilin family.[2]
FKBP1A (also known as FKBP12) is notable in humans for binding the immunosuppressant molecule tacrolimus (originally designated FK506), which is used in treating patients after organ transplant and patients with autoimmune disorders.[3] Tacrolimus has been found to reduce episodes of organ rejection over a related treatment, the drug ciclosporin, which binds cyclophilin.[4][5] Both the FKBP-tacrolimus complex and the cyclosporin-cyclophilin complex inhibit a phosphatase called calcineurin, thus blocking signal transduction in the T-lymphocyte transduction pathway.[6] This therapeutic role is not related to its prolyl isomerase activity. FKBP25 is a nuclear FKBP which non-specifically binds with DNA and has a role in DNA repair.[7]
- ^ Siekierka JJ, Hung SH, Poe M, Lin CS, Sigal NH (October 1989). "A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin". Nature. 341 (6244): 755–7. Bibcode:1989Natur.341..755S. doi:10.1038/341755a0. PMID 2477714. S2CID 4363530.
- ^ Balbach J, Schmid FX (2000). "Proline isomerization and its catalysis in protein folding". In Pain RH (ed.). Mechanisms of protein folding (2nd ed.). Oxford: Oxford University Press. pp. 212–237. ISBN 0-19-963789-X.
- ^ Wang T, Donahoe PK, Zervos AS (July 1994). "Specific interaction of type I receptors of the TGF-beta family with the immunophilin FKBP-12". Science. 265 (5172): 674–6. Bibcode:1994Sci...265..674W. doi:10.1126/science.7518616. PMID 7518616.
- ^ Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, Eigler FW, Heemann U, Pichlmayr R, Behrend M, Vanrenterghem Y, Donck J, van Hooff J, Christiaans M, Morales JM, Andres A, Johnson RW, Short C, Buchholz B, Rehmert N, Land W, Schleibner S, Forsythe JL, Talbot D, Pohanka E (August 1997). "Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group". Transplantation. 64 (3): 436–43. doi:10.1097/00007890-199708150-00012. PMID 9275110.
- ^ Prakash, Ajit; Rajan, Sreekanth; Yoon, Ho Sup (April 2016). "Crystal structure of the FK506 binding domain of human FKBP25 in complex with FK506". Protein Science. 25 (4): 905–910. doi:10.1002/pro.2875. ISSN 1469-896X. PMC 4941220. PMID 26749369.
- ^ Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL (August 1991). "Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes". Cell. 66 (4): 807–15. doi:10.1016/0092-8674(91)90124-H. PMID 1715244. S2CID 22094672.
- ^ Prakash, Ajit; Shin, Joon; Rajan, Sreekanth; Yoon, Ho Sup (2016-04-07). "Structural basis of nucleic acid recognition by FK506-binding protein 25 (FKBP25), a nuclear immunophilin". Nucleic Acids Research. 44 (6): 2909–2925. doi:10.1093/nar/gkw001. ISSN 1362-4962. PMC 4824100. PMID 26762975.