You can help expand this article with text translated from the corresponding article in German. (March 2021) Click [show] for important translation instructions.
|
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avigan (アビガン, Abigan), Avifavir,[1] Areplivir,[2] others |
| Other names | T-705, favipira, favilavir |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
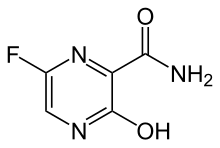
| Formula | C5H4FN3O2 |
| Molar mass | 157.104 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Favipiravir, sold under the brand name Avigan among others,[3] is an antiviral medication used to treat influenza in Japan.[4] It is also being studied to treat a number of other viral infections, including SARS-CoV-2.[4] Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.[5]
It is being developed and manufactured by Toyama Chemical (a subsidiary of Fujifilm) and was approved for medical use in Japan in 2014.[6] In 2016, Fujifilm licensed it to Zhejiang Hisun Pharmaceutical Co.[7] It became a generic drug in 2019.[citation needed]
- ^ Cite error: The named reference
Avifavirwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Areplivirwas invoked but never defined (see the help page). - ^ "Glenmark launches Covid-19 drug FabiFlu, priced at Rs 103 per tablet". Business Standard India. Press Trust of India. 20 June 2020.
- ^ a b Du YX, Chen XP (August 2020). "Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection". Clinical Pharmacology and Therapeutics. 108 (2): 242–247. doi:10.1002/cpt.1844. PMID 32246834.
- ^ Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, et al. (June 2009). "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections". Antiviral Research. 82 (3): 95–102. doi:10.1016/j.antiviral.2009.02.198. PMC 7127082. PMID 19428599.
- ^ Cite error: The named reference
Shi2020was invoked but never defined (see the help page). - ^ EJ Lane (22 June 2016). "Fujifilm in Avigan API license with Zhejiang Hisun Pharmaceuticals". Fierce Pharma. Retrieved 20 April 2020.