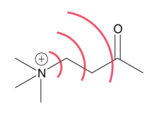
A field effect is the polarization of a molecule through space. The effect is a result of an electric field produced by charge localization in a molecule.[1] This field, which is substituent and conformation dependent, can influence structure and reactivity by manipulating the location of electron density in bonds and/or the overall molecule.[2] The polarization of a molecule through its bonds is a separate phenomenon known as induction.[3] Field effects are relatively weak, and diminish rapidly with distance, but have still been found to alter molecular properties such as acidity.[1]

- ^ a b Anslyn, Eric V., Dougherty, Dennis A. (2006). Modern physical organic chemistry. Sausalito, CA: University Science. ISBN 9781891389313. OCLC 55600610.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Wheeler, Steven E.; Houk, K. N. (2009). "Through-Space Effects of Substituents Dominate Molecular Electrostatic Potentials of Substituted Arenes". Journal of Chemical Theory and Computation. 5 (9): 2301–2312. doi:10.1021/ct900344g. ISSN 1549-9618. PMC 2806064. PMID 20161573.
- ^ L., Patrick, Graham (1997). Beginning organic chemistry. Oxford: Oxford University Press. ISBN 978-0198559368. OCLC 37293506.
{{cite book}}: CS1 maint: multiple names: authors list (link)