 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.007 |
| Chemical and physical data | |
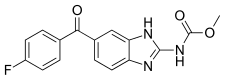
| Formula | C16H12FN3O3 |
| Molar mass | 313.288 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 243[1] °C (469 °F) |
| Solubility in water | 10.0 ± 0.4 × 10−6 μg/mL (pH 1.6); 0.29 ± 0.06 × 10−6 μg/mL (pH 6.5) [1] mg/mL (20 °C) |
| |
| |
| | |
Flubendazole is an anthelmintic, used both in humans and for veterinarian purposes. It is very close chemically to mebendazole, the only difference being an added fluorine group.[2]
- ^ a b Vasilev NA, Voronin AP, Surov AO, Perlovich GL (March 2023). "Influence of Co-amorphization on the Physical Stability and Dissolution Performance of an Anthelmintic Drug Flubendazole". Molecular Pharmaceutics. 20 (3): 1657–1669. doi:10.1021/acs.molpharmaceut.2c00873. PMID 36732935. S2CID 256546280.
- ^ Heyer F, Tourte-Schaeffer C, Ancelle T, Faurant C, Lapierre J (1 February 1982). "Le flubendazole : un progrès dans le traitement des helminthiases intestinales. A propos de 471 observations". Médecine et Maladies Infectieuses (in French). 12 (2): 57–61. doi:10.1016/S0399-077X(82)80047-4. ISSN 0399-077X.