 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
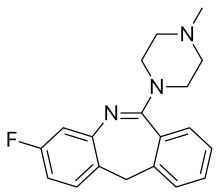
| Formula | C19H20FN3 |
| Molar mass | 309.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fluperlapine (NB 106-689), also known as fluoroperlapine, is a morphanthridine (11H-dibenzo[b,e]azepine) atypical antipsychotic with additional antidepressant and sedative effects. It was first synthesized in 1979, and then subsequently studied in animals and humans in 1984 and beyond,[1] but despite demonstrating efficacy in the treatment of a variety of medical conditions including schizophrenia,[2][3][4][5] psychosis associated with Parkinson's disease,[6] depressive symptoms, and dystonia,[7] it was never marketed.[1] This was perhaps due to its capacity for producing potentially life-threatening agranulocytosis, similarly to clozapine,[8] which it closely resembles both structurally and pharmacologically.
- ^ a b Ganellin CR, Triggle DJ, Macdonald F (1997). Dictionary of pharmacological agents. CRC Press. p. 916. ISBN 978-0-412-46630-4. Retrieved 15 September 2011.
- ^ Fischer-Cornelssen KA (1984). "Fluperlapine in 104 schizophrenic patients. Open multicenter trial". Arzneimittel-Forschung. 34 (1A): 125–30. PMID 6145428.
- ^ Woggon B, Angst J, Bartels M, Heinrich K, Hippius H, Koukkou M, et al. (1984). "Antipsychotic efficacy of fluperlapine. An open multicenter trial". Neuropsychobiology. 11 (2): 116–20. doi:10.1159/000118064. PMID 6148712.
- ^ Dieterle D, Eben E, Einhäupl K, Hippius H, Klein H, Rüther E, Schmauss M (March 1984). "The effect of fluperlapine in acute psychotic patients". Pharmacopsychiatry. 17 (2): 57–60. doi:10.1055/s-2007-1017408. PMID 6728910. S2CID 22264476.
- ^ Woggon B, Heinrich K, Küfferle B, Müller-Oerlinghausen B, Pöldinger W, Rüther E, Schied HW (1984). "Results of a multicenter AMDP study with fluperlapine in schizophrenic patients". Arzneimittel-Forschung. 34 (1A): 122–4. PMID 6145427.
- ^ Scholz E, Dichgans J (1985). "Treatment of drug-induced exogenous psychosis in parkinsonism with clozapine and fluperlapine". European Archives of Psychiatry and Neurological Sciences. 235 (1): 60–4. doi:10.1007/bf00380972. PMID 2864254. S2CID 23735955.
- ^ Pakkenberg H, Pedersen B (1985). "Medical treatment of dystonia". Dyskinesia. Psychopharmacology Supplementum. Vol. 2. pp. 111–7. doi:10.1007/978-3-642-70140-5_14. ISBN 978-3-642-70142-9. OCLC 10642795. PMID 2860654.
- ^ Lai WG, Gardner I, Zahid N, Uetrecht JP (March 2000). "Bioactivation and covalent binding of hydroxyfluperlapine in human neutrophils: implications for fluperlapine-induced agranulocytosis". Drug Metabolism and Disposition. 28 (3): 255–63. PMID 10681368.