 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 90% (oral), 70% (rectal)[1] |
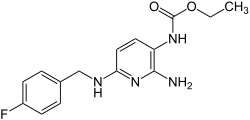
| Metabolism | Hepatic to 2-amino-3-acetylamino-6-(para-fluorobenzylamino) pyridine (which has 20-30% the analgesic potential of its parent compound), para-fluorohippuric acid[3] and a mercapturic acid metabolite, presumably formed from a glutathione adduct[4] |
| Elimination half-life | 6.5 hrs (average), 11.2-16.8 hrs (average 14 hrs) (elderly), 8.7-10.9 hrs (average 9.8 hrs) (in those with moderate-level renal impairment)[1] |
| Excretion | 72% of flupirtine and its metabolites appear in urine and 18% appear in feces[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.986 |
| Chemical and physical data | |
| Formula | C15H17FN4O2 |
| Molar mass | 304.325 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain[5] but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers.[6] In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.[7]
Flupirtine is a selective neuronal potassium channel opener (SNEPCO) that also has NMDA receptor antagonist and GABAA modulatory properties.[8]
It first became available in Europe in 1984 under the brand name Katadolon and after it went off patent many generic brands were introduced.[9]
- ^ a b Abrams SM, Baker LR, Crome P, White AS, Johnston A, Ankier SI, et al. (May 1988). "Pharmacokinetics of flupirtine in elderly volunteers and in patients with moderate renal impairment". Postgraduate Medical Journal. 64 (751): 361–363. doi:10.1136/pgmj.64.751.361. PMC 2428663. PMID 3200777.
- ^ Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE (2005). "Retigabine: chemical synthesis to clinical application". CNS Drug Reviews. 11 (1): 1–20. doi:10.1111/j.1527-3458.2005.tb00033.x. PMC 6741764. PMID 15867950.
- ^ Narang PK, Tourville JF, Chatterji DC, Gallelli JF (January 1984). "Quantitation of flupirtine and its active acetylated metabolite by reversed-phase high-performance liquid chromatography using fluorometric detection". Journal of Chromatography. 305 (1): 135–143. doi:10.1016/S0378-4347(00)83321-6. PMID 6707137.
- ^ Methling K, Reszka P, Lalk M, Vrana O, Scheuch E, Siegmund W, et al. (March 2009). "Investigation of the in vitro metabolism of the analgesic flupirtine". Drug Metabolism and Disposition. 37 (3): 479–493. doi:10.1124/dmd.108.024364. PMID 19074524. S2CID 5661841.
- ^ Harish S, Bhuvana K, Bengalorkar GM, Kumar T (April 2012). "Flupirtine: Clinical pharmacology". Journal of Anaesthesiology Clinical Pharmacology. 28 (2): 172–177. doi:10.4103/0970-9185.94833. PMC 3339720. PMID 22557738.
- ^ "Flupirtine-containing medicines". European Medicines Agency. November 21, 2013.
- ^ "European Medicines Agency - Human medicines - Flupirtine-containing medicinal products". www.ema.europa.eu. Archived from the original on 2022-01-21. Retrieved 2018-03-18.
- ^ Szelenyi I (March 2013). "Flupirtine, a re-discovered drug, revisited". Inflammation Research. 62 (3): 251–258. doi:10.1007/s00011-013-0592-5. PMID 23322112. S2CID 16535456.
- ^ Cite error: The named reference
brandswas invoked but never defined (see the help page).