 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Flovent, Flixotide, Flonase, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intranasal,[2] inhalation,[3] topical[4] |
| Drug class | Steroids and steroid derivatives |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism | Intranasal Liver (CYP3A4-mediated) |
| Elimination half-life | 10 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.129.097 |
| Chemical and physical data | |
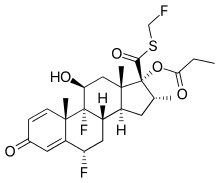
| Formula | C25H31F3O5S |
| Molar mass | 500.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluticasone propionate, sold under the brand names Flovent and Flonase among others, is a glucocorticoid steroid medication.[8] When inhaled it is used for the long term management of asthma and COPD.[8] In the nose it is used for hay fever and nasal polyps.[9][10] It can also be used for mouth ulcers.[11] It works by decreasing inflammation.
Common side effects when inhaled include upper respiratory tract infections, sinusitis, thrush, and cough.[8] Common side effects when used in the nose include nosebleeding and sore throat.[9] Unlike fluticasone furoate, which is approved in children as young as two years of age when used for allergies, fluticasone propionate is only approved for children four years and older.[12][13]
Fluticasone propionate was patented in 1980, and approved for medical use in 1990.[14] It is available as a generic medication.[10] In 2022, fluticasone was the 25th most commonly prescribed medication in the United States, with more than 22 million prescriptions.[15][16]
- ^ "Fluticasone Use During Pregnancy". Drugs.com. 9 January 2019. Archived from the original on 26 March 2019. Retrieved 31 January 2020.
- ^ Cite error: The named reference
Flonase Allergy Relief FDA labelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Flovent Diskus FDA labelwas invoked but never defined (see the help page). - ^ a b "Cutivate- fluticasone propionate lotion". DailyMed. 8 August 2018. Archived from the original on 20 February 2022. Retrieved 19 February 2022.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Respiratory health". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ "Flixonase Aqueous Nasal Spray - Summary of Product Characteristics (SmPC)". (emc). 25 October 2019. Archived from the original on 31 January 2020. Retrieved 31 January 2020.
- ^ a b c "Fluticasone Propionate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 February 2019. Retrieved 27 February 2019.
- ^ a b "Fluticasone Propionate eent Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 February 2019. Retrieved 27 February 2019.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 262, 1172. ISBN 9780857113382.
- ^ "Flixonase aqueous spray" (PDF). Sheffield Teaching Hospitals. June 2018. Archived from the original (PDF) on 25 September 2019. Retrieved 31 January 2020.
- ^ "Flonase Sensimist Allergy Relief- fluticasone furoate spray, metered". DailyMed. 30 May 2019. Archived from the original on 2 December 2020. Retrieved 4 February 2020.
- ^ "Veramyst- fluticasone furoate spray, metered". DailyMed. 1 March 2010. Archived from the original on 5 February 2020. Retrieved 4 February 2020.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495. Archived from the original on 28 November 2023. Retrieved 19 September 2020.
- ^ "The Top 300 of 2022". clincalc.com. Retrieved 19 August 2024.
- ^ "Fluticasone - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.