This article may be too technical for most readers to understand. (February 2013) |
Flux balance analysis (FBA) is a mathematical method for simulating metabolism in genome-scale reconstructions of metabolic networks. In comparison to traditional methods of modeling, FBA is less intensive in terms of the input data required for constructing the model. Simulations performed using FBA are computationally inexpensive and can calculate steady-state metabolic fluxes for large models (over 2000 reactions) in a few seconds on modern personal computers. The related method of metabolic pathway analysis seeks to find and list all possible pathways between metabolites.
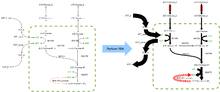
FBA finds applications in bioprocess engineering to systematically identify modifications to the metabolic networks of microbes used in fermentation processes that improve product yields of industrially important chemicals such as ethanol and succinic acid.[2] It has also been used for the identification of putative drug targets in cancer [3] and pathogens,[4] rational design of culture media,[5] and host–pathogen interactions.[6] The results of FBA can be visualized using flux maps similar to the image on the right, which illustrates the steady-state fluxes carried by reactions in glycolysis. The thickness of the arrows is proportional to the flux through the reaction.
FBA formalizes the system of equations describing the concentration changes in a metabolic network as the dot product of a matrix of the stoichiometric coefficients (the stoichiometric matrix S) and the vector v of the unsolved fluxes. The right-hand side of the dot product is a vector of zeros representing the system at steady state. Linear programming is then used to calculate a solution of fluxes corresponding to the steady state.
- ^ Forth, Thomas (2012). Metabolic systems biology of the malaria parasite. Leeds, UK: University of Leeds. ISBN 978-0-85731-297-6.
- ^ Ranganathan, Sridhar; Suthers, Patrick F.; Maranas, Costas D. (2010). "OptForce: An Optimization Procedure for Identifying All Genetic Manipulations Leading to Targeted Overproductions". PLOS Comput Biol. 6 (4): e1000744. Bibcode:2010PLSCB...6E0744R. doi:10.1371/journal.pcbi.1000744. PMC 2855329. PMID 20419153.
- ^ Lewis, NE; Abdel-Haleem, AM (2013). "The evolution of genome-scale models of cancer metabolism". Front. Physiol. 4: 237. doi:10.3389/fphys.2013.00237. PMC 3759783. PMID 24027532.
- ^ Raman, Karthik; Yeturu, Kalidas; Chandra, Nagasuma (2008). "targetTB: A Target Identification Pipeline for Mycobacterium tuberculosis Through an Interactome, Reactome and Genome-scale Structural Analysis". BMC Systems Biology. 2 (1): 109. doi:10.1186/1752-0509-2-109. PMC 2651862. PMID 19099550.
- ^ Yang, Hong; Roth, Charles M.; Ierapetritou, Marianthi G. (2009). "A rational design approach for amino acid supplementation in hepatocyte culture". Biotechnology and Bioengineering. 103 (6): 1176–1191. doi:10.1002/bit.22342. PMID 19422042. S2CID 13230467.
- ^ Raghunathan, Anu; Shin, Sookil; Daefler, Simon (2010). "Systems Approach to Investigating Host-pathogen Interactions in Infections with the Biothreat Agent Francisella. Constraints-based Model of Francisella tularensis". BMC Systems Biology. 4 (1): 118. doi:10.1186/1752-0509-4-118. PMC 2933595. PMID 20731870.