 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
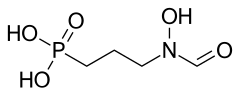
| Formula | C4H10NO5P |
| Molar mass | 183.100 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces.[1] It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM.[2] Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.[3][4]
- ^ Iguchi E, Okuhara M, Kohsaka M, Aoki H, Imanaka H (January 1980). "Studies on new phosphonic acid antibiotics. II. Taxonomic studies on producing organisms of the phosphonic acid and related compounds". The Journal of Antibiotics. 33 (1): 19–23. doi:10.7164/antibiotics.33.18. PMID 7372546.
- ^ Jawaid S, Seidle H, Zhou W, Abdirahman H, Abadeer M, Hix JH, van Hoek ML, Couch RD (December 2009). "Kinetic characterization and phosphoregulation of the Francisella tularensis 1-deoxy-D-xylulose 5-phosphate reductoisomerase (MEP synthase)". PLOS ONE. 4 (12): e8288. Bibcode:2009PLoSO...4.8288J. doi:10.1371/journal.pone.0008288. PMC 2788227. PMID 20011597.
- ^ Armstrong CM, Meyers DJ, Imlay LS, Freel Meyers C, Odom AR (September 2015). "Resistance to the antimicrobial agent fosmidomycin and an FR900098 prodrug through mutations in the deoxyxylulose phosphate reductoisomerase gene (dxr)". Antimicrobial Agents and Chemotherapy. 59 (9): 5511–9. doi:10.1128/AAC.00602-15. PMC 4538460. PMID 26124156.
- ^ Pines G, Oh EJ, Bassalo MC, Choudhury A, Garst AD, Fankhauser RG, Eckert CA, Gill RT (November 2018). "Genomic Deoxyxylulose Phosphate Reductoisomerase (DXR) Mutations Conferring Resistance to the Antimalarial Drug Fosmidomycin in E. coli". ACS Synthetic Biology. 7 (12): 2824–2832. doi:10.1021/acssynbio.8b00219. PMC 6928208. PMID 30462485.