This article's lead section contains information that is not included elsewhere in the article. (March 2023) |
| |||
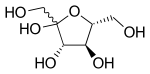
 Haworth projection of β-d-fructofuranose
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
D-arabino-Hex-2-ulose[3]
| |||
| Systematic IUPAC name
(3S,4R,5R)-1,3,4,5,6-Pentahydroxyhexan-2-one | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.303 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O6 | |||
| Molar mass | 180.156 g·mol−1 | ||
| Density | 1.694 g/cm3 | ||
| Melting point | 103 °C (217 °F; 376 K) | ||
| ~4000 g/L (25 °C) | |||
| −102.60×10−6 cm3/mol | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
675.6 kcal/mol (2,827 kJ/mol)[4] (Higher heating value) | ||
| Pharmacology | |||
| V06DC02 (WHO) | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
15000 mg/kg (intravenous, rabbit)[5] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fructose (/ˈfrʌktoʊs, -oʊz/), or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts most fructose and galactose into glucose for distribution in the bloodstream or deposition into glycogen. [6]
Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847.[7][8] The name "fructose" was coined in 1857 by the English chemist William Allen Miller.[9] Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars.[10] Fructose is found in honey, tree and vine fruits, flowers, berries, and most root vegetables.
Commercially, fructose is derived from sugar cane, sugar beets, and maize. High-fructose corn syrup is a mixture of glucose and fructose as monosaccharides. Sucrose is a compound with one molecule of glucose covalently linked to one molecule of fructose. All forms of fructose, including those found in fruits and juices, are commonly added to foods and drinks for palatability and taste enhancement, and for browning of some foods, such as baked goods. As of 2004, about 240,000 tonnes of crystalline fructose were being produced annually.[11]
Excessive consumption of sugars, including fructose, (especially from sugar-sweetened beverages) may contribute to insulin resistance, obesity, elevated LDL cholesterol and triglycerides, leading to metabolic syndrome. The European Food Safety Authority (EFSA) stated in 2011 that fructose may be preferable over sucrose and glucose in sugar-sweetened foods and beverages because of its lower effect on postprandial blood sugar levels,[12] while also noting the potential downside that "high intakes of fructose may lead to metabolic complications such as dyslipidaemia, insulin resistance, and increased visceral adiposity".[12][13] The UK's Scientific Advisory Committee on Nutrition in 2015 disputed the claims of fructose causing metabolic disorders, stating that "there is insufficient evidence to demonstrate that fructose intake, at levels consumed in the normal UK diet, leads to adverse health outcomes independent of any effects related to its presence as a component of total and free sugars."[14]
- ^ "Fructose". m-w.com. Merriam-Webster. Archived from the original on 19 April 2011. Retrieved 10 December 2014.
- ^ Levulose comes from the Latin word laevus, "left"; levulose is the old word for the most occurring isomer of fructose. D-fructose rotates plane-polarised light to the left, hence the name."Levulose". Archived from the original on 2009-10-08. Retrieved 2010-01-28..
- ^ "2-Carb-10". Archived from the original on 2023-06-18. Retrieved 2023-06-18.
- ^ CRC Handbook of Chemistry and Physics (49th ed.). 1968–69. p. D-186.
- ^ Chambers, Michael. "ChemIDplus – 57-48-7 – BJHIKXHVCXFQLS-UYFOZJQFSA-N – Fructose [USP:JAN] – Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. US National Institutes of Health. Archived from the original on 10 December 2014. Retrieved 10 December 2014.
- ^ https://diabetesjournals.org/care/article/25/2/353/23338/Increased-Fructose-Concentrations-in-Blood-and
- ^ Dubrunfaut (1847). "Sur une propriété analytique des fermentations alcoolique et lactique, et sur leur application à l'étude des sucres" [On an analytic property of alcoholic and lactic fermentations, and on their application to the study of sugars]. Annales de Chimie et de Physique (in French). 21: 169–178. Archived from the original on 2014-06-27. On page 174, Dubrunfaut relates the discovery and properties of fructose.
- ^ Fruton, J. S. (1974). "Molecules and Life – Historical Essays on the Interplay of Chemistry and Biology". Molecular Nutrition & Food Research. 18 (4). New York: Wiley-Interscience. doi:10.1002/food.19740180423. Archived from the original on 2021-02-28. Retrieved 2021-02-05.
- ^ Miller, William Allen (1857). "Part III. Organic Chemistry". Elements of Chemistry: Theoretical and Practical. London: John W. Parker and son. pp. 52, 57.
- ^ Hyvonen, L. & Koivistoinen, P (1982). "Fructose in Food Systems". In Birch, G.G. & Parker, K.J (eds.). Nutritive Sweeteners. London & New Jersey: Applied Science Publishers. pp. 133–144. ISBN 978-0-85334-997-6.
- ^ Wach, Wolfgang (2004). "fructose". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_047.pub2. ISBN 9783527303854.
- ^ a b "Scientific Opinion on the substantiation of health claims related to fructose and reduction of post-prandial glycaemic responses (ID 558) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (6). EFSA Panel on Dietetic Products, Nutrition and Allergies: 2223. 2011. doi:10.2903/j.efsa.2011.2223.
The Panel notes that these values support a significant decrease in post-prandial blood glucose responses when fructose replaces either sucrose or glucose.
- ^ Cite error: The named reference
efsa2-22was invoked but never defined (see the help page). - ^ "Carbohydrates and Health" (PDF). Williams Lea, Norwich, UK: UK Scientific Advisory Committee on Nutrition, Public Health England, TSO. 2015. Archived (PDF) from the original on 19 March 2016. Retrieved 1 April 2016.