 | |
| Clinical data | |
|---|---|
| Trade names | Omniscan |
| Other names | 2-[bis[2-(carboxylatomethyl-(methylcarbamoylmethyl)amino)ethyl]amino]acetate; gadolinium(+3) cation |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | negligible |
| Metabolism | not metabolized |
| Elimination half-life | 77.8 minutes |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
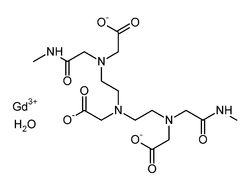
| Formula | C16H28GdN5O9 |
| Molar mass | 591.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gadodiamide, sold under the brand name Omniscan, is a gadolinium-based MRI contrast agent (GBCA), used in magnetic resonance imaging (MRI) procedures to assist in the visualization of blood vessels.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Omniscan- gadodiamide injection". DailyMed. Retrieved 29 August 2021.
- ^ "Active substance: gadodiamide" (PDF). List of nationally authorised medicinal products. European Medicine Agency. 14 January 2021.