 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| Chemical and physical data | |
| Formula | C11H15N5O3 |
| Molar mass | 265.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
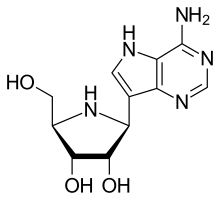
Galidesivir (BCX4430, immucillin-A) is an antiviral drug, an adenosine analog[1] (a type of nucleoside analog).[2] It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus.[3] Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.[4]
It also shows broad-spectrum antiviral effectiveness against a range of other RNA virus families, including bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, flaviviruses, and phleboviruses.[5] Galidesivir has been demonstrated to protect against both Ebola and Marburg viruses in both rodents and monkeys, even when administered up to 48 hours after infection,[1] and development for use in humans was then being fast-tracked due to concerns about the lack of treatment options for the 2013-2016 Ebola virus epidemic in West Africa.[6]
Galidesivir later showed efficacy against Zika virus in a mouse model.[7]
Galidesivir abrogates viremia in Zika virus–infected rhesus Macaques.[8]
Galidesivir is one of several antiviral drugs being tested for coronavirus disease 2019.[9]
On April 9, 2020, BioCryst opened enrollment into a randomized, double-blind, placebo-controlled clinical trial to assess the safety, clinical impact and antiviral effects of galidesivir in patients with COVID-19.[4]
- ^ a b Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, et al. (April 2014). "Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430". Nature. 508 (7496): 402–405. Bibcode:2014Natur.508..402W. doi:10.1038/nature13027. PMC 7095208. PMID 24590073.
- ^ Kamat SS, Burgos ES, Raushel FM (October 2013). "Potent inhibition of the C-P lyase nucleosidase PhnI by Immucillin-A triphosphate". Biochemistry. 52 (42): 7366–7368. doi:10.1021/bi4013287. PMC 3838859. PMID 24111876.
- ^ BioCryst Pharmaceuticals, Inc. (June 10, 2020). "Galidesivir Stops Zika Viral Replication in Primate Model". GlobeNewswire News Room (Press release).
- ^ a b Clinical trial number NCT03891420 for "A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19" at ClinicalTrials.gov
- ^ Westover JB, Mathis A, Taylor R, Wandersee L, Bailey KW, Sefing EJ, et al. (August 2018). "Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters". Antiviral Research. 156: 38–45. doi:10.1016/j.antiviral.2018.05.013. PMC 6035881. PMID 29864447.
- ^ Rodgers P (8 April 2014). "BioWar Lab Helping To Develop Treatment For Ebola". Forbes Magazine.
- ^ Julander JG, Siddharthan V, Evans J, Taylor R, Tolbert K, Apuli C, et al. (January 2017). "Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model". Antiviral Research. 137: 14–22. doi:10.1016/j.antiviral.2016.11.003. PMC 5215849. PMID 27838352.
- ^ Lim SY, Osuna CE, Best K, Taylor R, Chen E, Yoon G, et al. (June 2020). "A direct-acting antiviral drug abrogates viremia in Zika virus-infected rhesus macaques". Science Translational Medicine. 12 (547). doi:10.1126/scitranslmed.aau9135. PMC 7370316. PMID 32522808.
- ^ Duddu P (19 February 2020). "Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19". clinicaltrialsarena.com. Archived from the original on 19 February 2020. Retrieved 19 February 2020.