 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡænˈsaɪkləvɪər/ |
| Trade names | Cytovene; Cymevene; Vitrasert |
| Other names | gancyclovir; DHPG; 9-(1,3-dihydroxy-2-propoxymethyl)guanine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, by mouth, intravitreal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (oral) |
| Metabolism | guanylate kinase (CMV UL97 gene product) |
| Elimination half-life | 2.5–5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.155.403 |
| Chemical and physical data | |
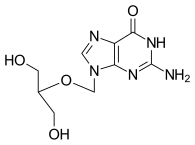
| Formula | C9H13N5O4 |
| Molar mass | 255.234 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 250 °C (482 °F) (dec.) |
| |
| |
| (verify) | |
Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat cytomegalovirus (CMV) infections.
Ganciclovir was patented in 1980 and approved for medical use in 1988.[4]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 13 July 2024.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495.