 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdʒɛntəˈmaɪsən/ |
| Trade names | Cidomycin, Genticyn, Garamycin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682275 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, eye drop, Intramuscular injection, Topical administration, ear drop |
| Drug class | Aminoglycoside antibiotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | limited bioavailability by mouth |
| Protein binding | 0–10% |
| Elimination half-life | 2 h |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.332 |
| Chemical and physical data | |
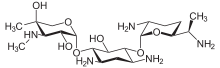
| Formula | C21H43N5O7 |
| Molar mass | 477.603 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gentamicin is an aminoglycoside antibiotic used to treat several types of bacterial infections.[4] This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others.[4] It is not effective for gonorrhea or chlamydia infections.[4] It can be given intravenously, by intramuscular injection, or topically.[4] Topical formulations may be used in burns or for infections of the outside of the eye.[5] It is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to.[6] The dose required should be monitored by blood testing.[4]
Gentamicin can cause inner ear problems and kidney problems.[4] The inner ear problems can include problems with balance and hearing loss.[4] These problems may be permanent.[4] If used during pregnancy, it can cause harm to the developing fetus.[4] However, it appears to be safe for use during breastfeeding.[7] Gentamicin is a type of aminoglycoside [4] and works by disrupting the ability of the bacteria to make proteins, which typically kills the bacteria.[4]
Gentamicin is naturally produced by the bacterium Micromonospora purpurea,[8][4] was patented in 1962, approved for medical use in 1964.[9] The antibiotic is collected from the culture of the Micromonospora by perforating the cell wall of the bacterium. Current research is underway to understand the biosynthesis of this antibiotic in an attempt to increase expression and force secretion of gentamicin for higher titer. Gentamicin is on the World Health Organization's List of Essential Medicines.[10] The World Health Organization classifies gentamicin as critically important for human medicine.[11] It is available as a generic medication.[12]
- ^ "Gentamicin Use During Pregnancy". Drugs.com. 28 February 2019. Retrieved 11 February 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Active substance: gentamicin (systemic use)" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 26 November 2020.
- ^ a b c d e f g h i j k l "Gentamicin sulfate". The American Society of Health-System Pharmacists. Archived from the original on 16 August 2015. Retrieved 15 August 2015.
- ^ Bartlett J (2013). Clinical Ocular Pharmacology (s ed.). Elsevier. p. 214. ISBN 9781483193915. Archived from the original on 22 December 2015.
- ^ Moulds R, Jeyasingham M (October 2010). "Gentamicin: a great way to start". Australian Prescriber. 33 (5): 134–135. doi:10.18773/austprescr.2010.062.
- ^ "Gentamicin use while breastfeeding". Archived from the original on 6 September 2015. Retrieved 15 August 2015.
- ^ Weinstein MJ, Luedemann GM, Oden EM, Wagman GH, Rosselet JP, Marquez JA, et al. (July 1963). "Gentamicin, a new antibiotic complex from Micromonospora". Journal of Medicinal Chemistry. 6 (4): 463–464. doi:10.1021/jm00340a034. PMID 14184912.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 507. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2019). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. hdl:10665/312266. ISBN 9789241515528. License: CC BY-NC-SA 3.0 IGO.
- ^ Burchum J (2014). Lehne's pharmacology for nursing care. Elsevier Health Sciences. p. 1051. ISBN 9780323340267. Archived from the original on 11 March 2016.