 | |
| Clinical data | |
|---|---|
| Trade names | Mavyret (combination with pibrentasvir) |
| Other names | ABT-493 |
| Routes of administration | By mouth |
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | 97.5% |
| Metabolism | CYP3A |
| Elimination half-life | 6 hours |
| Excretion | Faeces (92.1%), urine (0.7%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
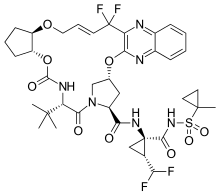
| Formula | C38H46F4N6O9S |
| Molar mass | 838.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Glecaprevir (INN,[1]) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals.[2] It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance in vitro.[3]
On 19 December 2016, AbbVie submitted a new drug application to the U.S. Food and Drug Administration for the glecaprevir/pibrentasvir (trade name Mavyret) regimen for the treatment of all major genotypes (1–6) of chronic hepatitis C. On 3 August 2017 the FDA approved the combination for hepatitis C treatment.[4] In Europe, it was approved on 17 August 2017 for the same indication, under the trade name Maviret.[5]
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 76" (PDF). World Health Organization. p. 503. Retrieved 25 February 2017.
- ^ Notman, Nina (August 14, 2023). "ACS names its 2023 Heroes of Chemistry". Chemical & Engineering News. Vol. 101, no. 26. Archived from the original on 2024-03-16. Retrieved 2024-04-04.
- ^ Lawitz EJ, O'Riordan WD, Asatryan A, Freilich BL, Box TD, Overcash JS, et al. (December 2015). "Potent Antiviral Activities of the Direct-Acting Antivirals ABT-493 and ABT-530 with Three-Day Monotherapy for Hepatitis C Virus Genotype 1 Infection". Antimicrobial Agents and Chemotherapy. 60 (3): 1546–55. doi:10.1128/AAC.02264-15. PMC 4775945. PMID 26711747.
- ^ "AbbVie Submits New Drug Application to U.S. FDA for its Investigational Regimen of Glecaprevir/Pibrentasvir (G/P) for the Treatment of All Major Genotypes of Chronic Hepatitis C." AbbVie Inc. North Chicago, Illinois, U.S.A. December 19, 2016. Archived from the original on 20 August 2017. Retrieved 25 February 2017.
- ^ "Maviret: EPAR – Summary for the public" (PDF). European Medicines Agency. 2017-08-17. Archived from the original (PDF) on 2017-10-19. Retrieved 2017-10-20.