 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diamicron, Diaprel, Azukon, others[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 10.4 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.221 |
| Chemical and physical data | |
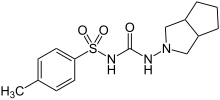
| Formula | C15H21N3O3S |
| Molar mass | 323.41 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 180 to 182 °C (356 to 360 °F) |
| |
| |
| (verify) | |
Gliclazide, sold under the brand name Diamicron among others, is a sulfonylurea type of anti-diabetic medication, used to treat type 2 diabetes.[7] It is used when dietary changes, exercise, and weight loss are not enough.[4] It is taken by mouth.[7]
Side effect may include low blood sugar, vomiting, abdominal pain, rash, and liver problems.[4][7] Use by those with significant kidney problems or liver problems or who are pregnant is not recommended.[7][4] Gliclazide is in the sulfonylurea family of medications.[7] It works mostly by increasing the release of insulin.[7]
Gliclazide was patented in 1966 and approved for medical use in 1972.[8] It is on the World Health Organization's List of Essential Medicines.[9] It is not available for sale in the United States.[10]
- ^ "Gliclazide - Drugs.com". www.drugs.com. Archived from the original on 27 December 2016. Retrieved 27 December 2016.
- ^ "Gliclazide GH MR, Gliclazide LAPL MR, Gliclazide Lupin MR (Lupin Australia Pty Limited)". Therapeutic Goods Administration (TGA). 5 December 2022. Archived from the original on 18 March 2023. Retrieved 7 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Archived from the original on 29 March 2024. Retrieved 3 April 2024.
- ^ a b c d "Gliclazide Accord-UK 30mg Prolonged-release Tablets - Summary of Product Characteristics (SmPC)". (emc). 12 February 2021. Archived from the original on 22 September 2022. Retrieved 30 December 2021.
- ^ "Diamicron 30 mg MR Tablets - Summary of Product Characteristics (SmPC)". (emc). 11 May 2020. Archived from the original on 23 December 2023. Retrieved 29 December 2021.
- ^ "Dacadis MR 30mg Modified Release Tablets - Summary of Product Characteristics (SmPC)". (emc). 15 July 2020. Archived from the original on 8 November 2023. Retrieved 29 December 2021.
- ^ a b c d e f British National Formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 474. ISBN 9780857111562.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495. Archived from the original on 27 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Gliclazide Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 27 December 2016. Retrieved 27 December 2016.