| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pent-2-enedioic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6O4 | |||
| Molar mass | 130.099 g/mol | ||
| Appearance | Colorless solid | ||
| Melting point | 137 to 139 °C (279 to 282 °F; 410 to 412 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
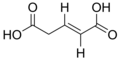
trans-Glutaconic acid is an organic compound with formula HO2CCH=CHCH2CO2H. This dicarboxylic acid exists as a colorless solid and is related to the saturated chemical glutaric acid, HO2CC(CH2)3CO2H. Esters and salts of glutaconic acid are called glutaconates.
Glutaconate bound to coenzyme A, glutaconyl-CoA, is an intermediate in lysine metabolism.[1]
- ^ Voet, Donald; Voet, Judith G. (2011). Biochemistry (4 ed.). Hoboken, NJ: Wiley. pp. 1040–1041. ISBN 978-0-470-57095-1.