| |
| Names | |
|---|---|
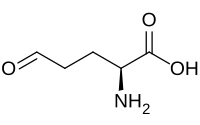
| IUPAC name
(2S)-2-Amino-5-oxopentanoic acid
| |
| Other names
L-Glutamate gamma-semialdehyde; gamma-Glutamyl semialdehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9NO3 | |
| Molar mass | 131.131 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine (via ornithine),[1][2] as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the reduction of glutamyl-5-phosphate by glutamate-5-semialdehyde dehydrogenase.
Reduction of glutamic acid semialdehyde with sodium borohydride gives hydroxyaminovaleric acid.[3]
- ^ Baich A (1971). "The Biosynthesis of Proline in Escherichia coli: Phosphate-Dependent Glutamate-semialdehyde Dehydrogenase (NADP), the Second Enzyme in the Pathway". Biochim. Biophys. Acta. 244 (1): 129–34. doi:10.1016/0304-4165(71)90129-2. PMID 4399189.
- ^ Voet, Donald (2011). Biochemistry. Judith G. Voet (4th ed.). Hoboken, NJ: John Wiley & Sons. ISBN 978-0-470-57095-1. OCLC 690489261.
- ^ Requena, J. R.; Levine, R. L.; Stadtman, E. R. (2003). "Recent Advances in the Analysis of Oxidized Proteins". Amino Acids. 25 (3–4): 221–226. doi:10.1007/s00726-003-0012-1. PMID 14661085. S2CID 28837698.