 | |
| Clinical data | |
|---|---|
| Trade names | Robinul, Cuvposa, Seebri, others |
| Other names | glycopyrrolate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602014 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, inhalation, topical, injection, subcutaneous |
| Drug class | Antimuscarinic (peripherally-selective) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 0.6–1.2 hours |
| Excretion | 85% Kidney, unknown amount in the bile |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.990 |
| Chemical and physical data | |
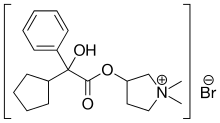
| Formula | C19H28BrNO3 |
| Molar mass | 398.341 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| | |
Glycopyrronium bromide is a medication of the muscarinic anticholinergic group.[7] It does not cross the blood–brain barrier and consequently has few to no central effects. It is given by mouth,[8] via intravenous injection, on the skin,[9] and via inhalation.[4][5][6] It is a synthetic quaternary ammonium compound.[2] The cation, which is the active moiety, is called glycopyrronium (INN)[10] or glycopyrrolate (USAN).
The most common side effects include irritability, flushing, nasal congestion, reduced secretions in the airways, dry mouth, constipation, diarrhea, nausea and vomiting, and urinary retention.[7]
In September 2012, glycopyrronium was approved for medical use in the European Union.[4] In June 2018, glycopyrronium was approved by the U.S. Food and Drug Administration (FDA) to treat excessive underarm sweating, becoming the first drug developed specifically to reduce excessive sweating.[11] It is on the World Health Organization's List of Essential Medicines.[12]
- ^ "Neurological therapies". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ a b "Robinul- glycopyrrolate tablet Robinul Forte- glycopyrrolate tablet". DailyMed. 1 June 2021. Retrieved 20 June 2022.
- ^ "Dartisla ODT- glycopyrrolate orally disintegrating tablets tablet, orally disintegrating". DailyMed. 9 December 2021. Retrieved 20 June 2022.
- ^ a b c Cite error: The named reference
Seebri Breezhaler EPARwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Tovanor Breezhaler EPARwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Enurev Breezhaler EPARwas invoked but never defined (see the help page). - ^ a b c "Sialanar EPAR". European Medicines Agency. 17 September 2018. Retrieved 29 January 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Glycopyrrolate Oral Inhalation". MedlinePlus. Archived from the original on 17 August 2021. Retrieved 20 June 2022.
- ^ "Glycopyrronium Topical". MedlinePlus. Archived from the original on 17 August 2021. Retrieved 20 June 2022.
- ^ Bajaj V, Langtry JA (July 2007). "Use of oral glycopyrronium bromide in hyperhidrosis". The British Journal of Dermatology. 157 (1): 118–121. doi:10.1111/j.1365-2133.2007.07884.x. PMID 17459043. S2CID 29080876.
- ^ "FDA OKs first drug made to reduce excessive sweating". AP News. Archived from the original on 2018-07-02. Retrieved 2018-07-02.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.