 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zoladex, others |
| Other names | D-Ser(But)6Azgly10-GnRH |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601002 |
| Routes of administration | Implant |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 27.3% |
| Elimination half-life | 4–5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.212.024 |
| Chemical and physical data | |
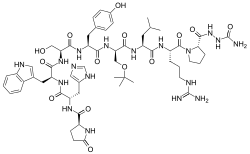
| Formula | C59H84N18O14 |
| Molar mass | 1269.433 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Goserelin, sold under the brand name Zoladex among others, is a medication which is used to suppress production of the sex hormones (testosterone and estrogen), particularly in the treatment of breast cancer and prostate cancer.[2][3] It is an injectable gonadotropin releasing hormone agonist (GnRH agonist).
Structurally, it is a decapeptide. It is the natural GnRH decapeptide with two substitutions to inhibit rapid degradation.
Goserelin stimulates the production of the sex hormones testosterone and estrogen in a non-pulsatile (non-physiological) manner. This causes the disruption of the endogenous hormonal feedback systems, resulting in the down-regulation of testosterone and estrogen production.
It was patented in 1976 and approved for medical use in 1987.[4] It is on the World Health Organization's List of Essential Medicines.[5]
- ^ "Product monograph brand safety updates". Health Canada. 6 June 2024. Retrieved 8 June 2024.
- ^ Dictionary of Organic Compounds. CRC Press. 1996. pp. 3372–. ISBN 978-0-412-54090-5.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 136–. ISBN 978-94-011-4439-1.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 514. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.