| Graphene | |
|---|---|
 | |
| Material type | Allotrope of carbon |
| Chemical properties | |
| Chemical formula | C |
| Mechanical properties | |
| Young's modulus (E) | ≈1 TPa |
| Tensile strength (σt) | 130 GPa |
| Thermal properties | |
| Thermal conductivity (k) | 5300 W⋅m−1⋅K−1 |
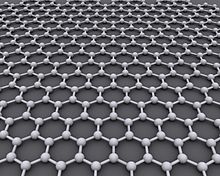
Graphene (/ˈɡræfiːn/)[1] is a carbon allotrope consisting of a single layer of atoms arranged in a honeycomb planar nanostructure.[2][3] The name "graphene" is derived from "graphite" and the suffix -ene, indicating the presence of double bonds within the carbon structure.
Graphene is known for its exceptionally high tensile strength, electrical conductivity, transparency, and being the thinnest two-dimensional material in the world.[4] Despite the nearly transparent nature of a single graphene sheet, graphite (formed from stacked layers of graphene) appears black because it absorbs all visible light wavelengths.[5][6] On a microscopic scale, graphene is the strongest material ever measured.[7][8]

The existence of graphene was first theorized in 1947 by Philip R. Wallace during his research on graphite's electronic properties.[9] In 2004, the material was isolated and characterized by Andre Geim and Konstantin Novoselov at the University of Manchester[10][11] using a piece of graphite and adhesive tape.[12] In 2010, Geim and Novoselov were awarded the Nobel Prize in Physics for their "groundbreaking experiments regarding the two-dimensional material graphene".[13] While small amounts of graphene are easy to produce using the method by which it was originally isolated, attempts to scale and automate the manufacturing process for mass production have had limited success due to cost-effectiveness and quality control concerns.[14][15] The global graphene market was $9 million in 2012,[16] with most of the demand from research and development in semiconductors, electronics, electric batteries,[17] and composites.
The IUPAC (International Union of Pure and Applied Chemistry) advises using the term "graphite" for the three-dimensional material and reserving "graphene" for discussions about the properties or reactions of single-atom layers.[18] A narrower definition, of "isolated or free-standing graphene", requires that the layer be sufficiently isolated from its environment,[19] but would include layers suspended or transferred to silicon dioxide or silicon carbide.[20]
- ^ Cite error: The named reference
camdicwas invoked but never defined (see the help page). - ^ Cite error: The named reference
geim2007was invoked but never defined (see the help page). - ^ Cite error: The named reference
peres2009was invoked but never defined (see the help page). - ^ Pike, Jared (2023). "Is graphene the best heat conductor ever? Purdue researchers investigate with four-phonon scattering". Purdue University Mechanical Engineering News. Archived from the original on 4 March 2024. Retrieved 1 October 2024.
- ^ a b Cite error: The named reference
nair2008was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
zhu2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
lee2008was invoked but never defined (see the help page). - ^ Cite error: The named reference
cao2020was invoked but never defined (see the help page). - ^ "Graphene: A Complete Chemical History". ACS Material. 20 September 2019. Retrieved 1 October 2024.
In 1947, the existence of graphene was theorized by Philip R Wallace as an attempt to understand electronic properties of 3D graphite. He did not use the term "graphene", but instead referred to it as a "single hexagonal layer."
- ^ Cite error: The named reference
novo2004was invoked but never defined (see the help page). - ^ Cite error: The named reference
aps2009was invoked but never defined (see the help page). - ^ "Discovery of graphene - Graphene - The University of Manchester". www.graphene.manchester.ac.uk. Retrieved 16 October 2024.
- ^ "The Nobel Prize in Physics 2010". Nobel Foundation. Archived from the original on 22 May 2020. Retrieved 1 September 2021.
- ^ "Mass-Producing Graphene". American Scientist. 6 April 2018. Retrieved 16 October 2024.
- ^ Joshi, Rita (8 April 2024). "Can Graphene Be Mass Produced?". AZoNano. Retrieved 16 October 2024.
- ^ Cite error: The named reference
azon2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
mrmak2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
IUPAC2009was invoked but never defined (see the help page). - ^ Cite error: The named reference
geim2009awas invoked but never defined (see the help page). - ^ Cite error: The named reference
ried2009was invoked but never defined (see the help page).