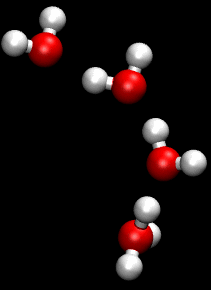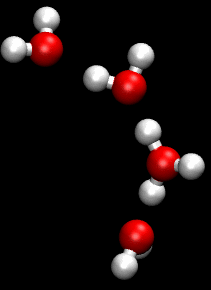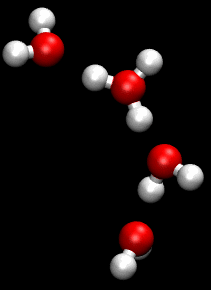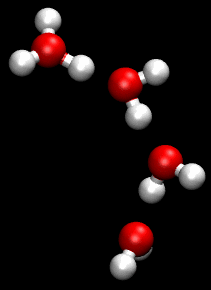
The Grotthuss mechanism (also known as proton jumping) is a model for the process by which an 'excess' proton or proton defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation and concomitant cleavage of covalent bonds involving neighboring molecules.
In his 1806 publication “Theory of decomposition of liquids by electrical currents”, Theodor Grotthuss proposed a theory of water conductivity.[1] Grotthuss envisioned the electrolytic reaction as a sort of ‘bucket line’ where each oxygen atom simultaneously passes and receives a single hydrogen ion. It was an astonishing theory to propose at the time, since the water molecule was thought to be OH, not H2O, and the existence of ions was not fully understood. On its 200th anniversary, his article was reviewed by Cukierman.[2]
Although Grotthuss was using an incorrect empirical formula of water, his description of the passing of protons through the cooperation of neighboring water molecules proved prescient.
Lemont Kier suggested that proton hopping may be an important mechanism for nerve transduction.[3]
- ^ de Grotthuss, C.J.T. (1806). "Sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique". Ann. Chim. 58: 54–73.
- ^ Cukierman, Samuel (2006). "Et tu Grotthuss!". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1757 (8): 876–8. doi:10.1016/j.bbabio.2005.12.001. PMID 16414007.
- ^ Kier, Lemont B. (2016). "Proton Hopping as the Nerve Conduction Message". Current Computer-Aided Drug Design. 12 (4): 255–258. doi:10.2174/1573409912666160808092011. ISSN 1875-6697. PMID 27503744.