| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5,7-Tetranitro-1,3,5,7-tetrazocane | |
| Other names
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.018.418 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8N8O8 | |
| Molar mass | 296.155 g/mol |
| Density | 1.91 g/cm3, solid |
| Melting point | 276 to 286 °C (529 to 547 °F; 549 to 559 K) |
| Explosive data | |
| Shock sensitivity | Low |
| Friction sensitivity | Low |
| Detonation velocity | 9100 m/s |
| RE factor | 1.70 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Explosive |
| GHS labelling: | |
 
| |
| Danger | |
| H201, H205, H241, H301, H304, H311, H319 | |
| P210, P250, P280, P370+P380, P372, P373 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
HMX, also called octogen, is a powerful and relatively insensitive nitroamine high explosive chemically related to RDX. The compound's name is the subject of much speculation, having been variously listed as High Melting Explosive, High-velocity Military Explosive, or High-Molecular-weight RDX.[1]
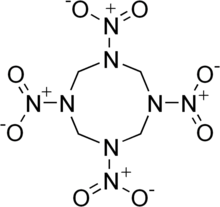
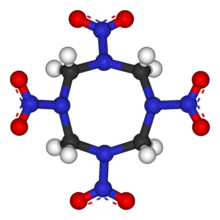
The molecular structure of HMX consists of an eight-membered ring of alternating carbon and nitrogen atoms, with a nitro group attached to each nitrogen atom. Because of its high mass-specific enthalpy of formation, it is one of the most potent chemical explosives manufactured, although a number of newer ones, including HNIW and ONC, are more powerful.
- ^ Cooper, Paul W., Explosives Engineering, New York: Wiley-VCH, 1996. ISBN 0-471-18636-8
