| Halobacterium | |
|---|---|
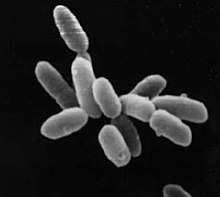
| |
| Halobacterium sp. strain NRC-1, each cell about 5 μm in length | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Halobacterium (Elazari-Volcani 1940) Elazari-Volcani 1957 non Schoop
|
| Type species | |
| Halobacterium salinarum (Harrison & Kennedy 1922) Elazari-Volcani 1957
| |
| Species | |
| |
| Synonyms | |
| |
Halobacterium (common abbreviation Hbt.) is a genus in the family Halobacteriaceae.[1]
The genus Halobacterium ("salt" or "ocean bacterium") consists of several species of Archaea with an aerobic metabolism which requires an environment with a high concentration of salt; many of their proteins will not function in low-salt environments. They grow on amino acids in their aerobic conditions. Their cell walls are also quite different from those of bacteria, as ordinary lipoprotein membranes fail in high salt concentrations. In shape, they may be either rods or cocci, and in color, either red or purple. They reproduce using binary fission (by constriction), and are motile. Halobacterium grows best in a 42 °C environment. The genome of an unspecified Halobacterium species, sequenced by Shiladitya DasSarma, comprises 2,571,010 bp (base pairs) of DNA compiled into three circular strands: one large chromosome with 2,014,239 bp, and two smaller ones with 191,346 and 365,425 bp. This species, called Halobacterium sp. NRC-1, has been extensively used for postgenomic analysis. Halobacterium species can be found in the Great Salt Lake, the Dead Sea, Lake Magadi, and any other waters with high salt concentration. Purple Halobacterium species owe their color to bacteriorhodopsin, a light-sensitive protein which provides chemical energy for the cell by using sunlight to pump protons out of the cell. The resulting proton gradient across the cell membrane is used to drive the synthesis of the energy carrier ATP. Thus, when these protons flow back in, they are used in the synthesis of ATP (this proton flow can be emulated with a decrease in pH outside the cell, causing a flow of H+ ions). The bacteriorhodopsin protein is chemically very similar to the light-detecting pigment rhodopsin, found in the vertebrate retina.
- ^ See the NCBI webpage on Halobacterium. Data extracted from the "NCBI taxonomy resources". National Center for Biotechnology Information. Retrieved 2007-03-19.